Preventing Negative Shifts in Gut Microbiota with Cannabis Therapy: Implications for Colorectal Cancer_Juniper Publishers
ADVANCED RESEARCH IN GASTROENTEROLOGY & HEPATOLOGY JUNIPER PUBLISHERS
Authored by Myron R Szewczuk
Abstract
Despite the controversies surrounding the therapeutic use of cannabis in the treatment of several medical conditions, the fact remains that there is a scientific rationale for its use, particularly in the treatment of colorectal cancer (CRC). Constituents of cannabis such as CBD have demonstrated anti-cancer effects via endocannabinoid signaling. However, an additional positive aspect of cannabis use that may be overlooked is its potential role in preventing imbalances of the gut microbiota. Not only is this important for the treatment of several gastrointestinal disorders such as inflammatory bowel disease (IBD) and obesity, but it also has implications for the treatment of CRC. The impact of the endocannabinoid system on gut microbiota is a relatively new and emerging field wherein the interplay between cannabinoids and metabolic syndrome has been the focus thus far. This review aims to introduce the link between cannabis, obesity and CRC through alterations to gut microbiota, which is an exciting new direction of research. Here, we will outline the current understanding of the mechanism of action of cannabis on the endocannabinoid system as well as its therapeutic potential. We will also discuss the connection between imbalances in gut microbiota, the action of cannabis in correcting this occurrence and the potentially overlooked positive implications of preventing further negative changes to gut microbiota for the initiation of CRC.
Keywords: Gut microbiota; Colorectal cancer; Cannabis; Marijuana; Metabolic syndrome
Introduction
Recently, with the increased interest in the therapeutic potential of MM, or cannabis (used interchangeably hereafter), reports have suggested the existence of a relationship between the endocannabinoid system, a naturally occurring system in humans, and gastrointestinal (GI) tract function, proposing that cannabinoids may also impact the gut microbiota [1]. The gut microbiota is a collection of microorganisms that are involved in several homeostatic and immune functions [2]. Although microbiota composition is influenced by host genotype and physiology, additional factors such as diet, antibiotic use, smoking, exercise and stress have also been implicated in contributing to the composition of individual gut microbiota [1,3]. Since relative levels of gut microorganisms have been previously associated with playing a crucial role in the initiation and progression of colorectal cancer (CRC) [2], the potential connection between the modulatory role of cannabis, particularly as an anti-cancer agent [4], is perhaps not surprising. A new field of research has emerged that has linked the interactions of cannabis in rebalancing the GI tract microbiota and the potential preventative action on the development of CRC. Here, we will discuss how phytocannabinoids like cannabis may alter GI tract characteristics and gut microbiota composition. We will also cover the implications of the modulatory effects of cannabis on the GI tract as they specifically relate to CRC.
Endocannabinoids and the gut
Increased endocannabinoid system tone has been observed in intestinal inflammation and obesity [5] and includes heightened endocannabinoid levels in plasma and adipose tissue as well as increased expression of the cannabinoid 1(CBD) receptor [6]. It has been reported that increased levels of endocannabinoids acting primarily through the CB1 receptor serve a protective role against epithelial damage and increased motility characteristic of intestinal inflammation [5]. These properties extend to cannabis as well, as it acts on the endocannabinoid system in a similar manner to endogenous endocannabinoids via CB1 and CB2 receptor signaling [6,7].
Cannabis is a phytocannabinoid acting on the endocannabinoid system, an inherent communication network of cannabinoid receptors and endogenous ligands ubiquitously expressed in the body [4]. The most commonly studied active agents of cannabis include psychoactive Δ9-tetrahydrocannabinol (THC) and non-psychoactive cannabidiol (CBD), both of which act on CB1 and CB2 receptors. Recent studies have reported that cannabinoids modulate cholinergic neurotransmission within the endocannabinoid system [8]. The CB1 receptor is primarily expressed in the nervous system, found on neuronal cells, the brain, endocrine tissues and other peripheral tissues [7]. In contrast, CB2 receptors are more restricted in their distribution, and are mainly found on some immune cells [9]. In the gut, the CB1 receptor is implicated in enteric nervous system function [8,10,11], expressed on epithelial cells, submucosal neurons, and myenteric neurons [5], providing the means by which cannabinoids modulate intestinal motility [11]. Activation of the CB1 receptor on epithelial cells promotes wound healing, while the corresponding receptor on submucosal neurons is associated with decreased secretions. Interestingly, CB1 receptors on the myenteric nerve plexus were found to inhibit motility in mouse models of intestinal inflammation [5]. Due to the expression of CB2 receptors primarily on immune cells, their activation has been associated with the inhibition of pro-inflammatory cytokines such as tumor necrosis factor a (TNF-α) [5]. Taken together, the presence of CB1 and CB2 receptors in the gut wall has been implicated in the regulation of food intake, GI tract motility, intestinal inflammation, cell proliferation, nausea and emesis [10]. Bearing in mind that alterations in gut microbiota have been associated with changes to endothelial membrane integrity and inflammation [2], it is perhaps not surprising that there may be a link between cannabinoids and gut microbiota composition with respect to slowing the initiation of CRC.
Gut microbiota composition
Human gut microbiota consists of four main phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria [12]. Of these, Firmicutes and Bacteroidetes are of particular interest due to their proposed role in obesity and progression of CRC [12,13]. The microbiota profile of obese individuals typically displays a high Firmicutes:Bacteroidetes ratio [14]. This was confirmed by Ley et al. [15] showing a 50% reduction in Bacteroidetes and a corresponding increase in Firmicutes. Low calorie diet [16] and prebiotic fiber supplementation [17] are known to restore this ratio, and more recently, long-term THC intake has demonstrated its ability to halt this shift in microbiota ratio [13].
A closer look at precisely how THC prevents further gut microbiota dysbiosis reveals that vehicle-treated diet-induced obese (DIO) mice receiving a high-fat diet demonstrated an increased Firmicutes:Bacteroidetes ratio, while THC-treated DIO mice did not experience a shift in this ratio [13]. In addition to the change in microbiota composition, THC intake was also accompanied by weight loss in DIO mice [13], as an increase in Bacteroidetes has been associated with weight loss [16].Although seemingly counterintuitive, the weight loss caused by THC is due to its partial agonist effect and only occurs under high endocannabinoid tone conditions [7,13]. THC acts at the cannabinoid receptors, but at a decreased efficacy compared to full endogenous cannabinoid agonists [13]. As a result, THC blocks the function of endogenous CB1 receptor agonists that would otherwise promote hunger. Beyond that, the exact mechanism behind THC's ability to produce weight-loss under high endocannabinoid tone remains to be elucidated. Moreover, it remains to be determined whether the weight loss associated with THC administration in DIO mice is a consequence of changes to gut microbiota, or vice versa.
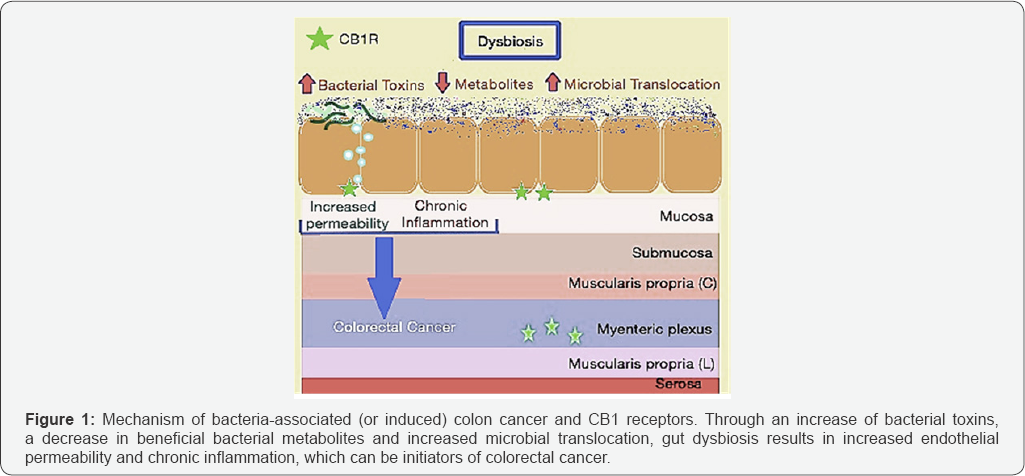
Given that obesity is a risk-factor for colorectal cancer, it is perhaps not surprising that an increased Firmicutes: Bacteroidetes ratio has also been correlated with disease progression of colorectal cancer [12]. Gao et al. [18] eloquently described the link between microbiota dysbiosis to sporadic CRC, with CRC patients exhibiting markedly different microbial structures compared to healthy individuals. Cancerous gut tissue contained significantly higher levels of Firmicutes (63.46%) when compared to healthy tissue (30.54%), which mirrors the elevated Firmicutes shown to accompany obesity in DIO mice. Additionally, cancerous tissue contained lower levels of Bacteroidetes (12.77%) in comparison to healthy tissue (19.1%) [13]. This is particularly interesting because Gram-negative bacteria in the Bacteroidetes phylum predominantly produce short-chain fatty acids (SCFAs) acetate and propionate, both of which are closely related to obesity by playing roles in cholesterol synthesis and reducing intake of food, respectively [19]. Preventing the Firmicutes: Bacteroidetes ratio from increasing may be of critical importance as colon cancer is thought to be bacterially induced (or associated) [12] in accordance with one of the major mechanisms of colon cancer initiation. According to this model, gut microbiota dysbiosis leads to increased permeability, abnormal immune activation, chronic inflammation and hyperproliferation, all of which contribute directly to colorectal cancer initiation and progression [12], as briefly summarized in Figure 1 below. This is accomplished by means of toxic bacterial products, expression of fewer beneficial bacterial metabolites, disruptions to tissue bARGHiers and translocation of microbes, which are all common under conditions of dysbiosis [12]. As a result, rebalance of gut microbiota dysbiosis early in disease onset may be critical to preventing the progression of colorectal cancer.
Together, these data suggest that by virtue of interfering with the appetite-stimulating property of endocannabinoids, cannabis may prove to be an alternate therapeutic approach in promoting weight loss in obese individuals, thereby lowering their risk of developing CRC. Since cannabis has a well-established role in maintaining the integrity of the endothelial membrane and serving as an anti-inflammatory, the added benefit of using cannabis to halt negative changes to gut microbiota in obese individuals may be a novel way to target CRC prevention.
Cannabis: a guardian of endothelial membrane integrity and an anti-inflammatory agent
It has been previously reported that perturbation in gut microbiota can result in increased tight junction permeability that can initiate an inflammatory cascade [2]. This occurs when breaches to the epithelial bARGHier result in commensal bacteria and pathogens infiltrating the mucosal and submucosal bARGHier [12]. Consequently, Toll-like receptors (TLRs) and Nod-like receptors (NLRs) can recognize select pathogenic molecular motifs [2]. This detection results in the activation of the MAPK, NF-ĸB, and P13K/AKT signaling pathways whose downstream effectors include pro-inflammatory cytokines [2,20]. TNF-α is particularly pertinent under intestinal inflammatory conditions on account of its induction of interleukein 8 (IL-8) in human colonic epithelial cells. Both TNF-α and IL-8 play key roles in the maintenance of intestinal immune homeostasis [5]. Under inflammatory conditions, immune cell activation, cytokine and chemokine production, proliferation and recruitment result in the infiltration of additional immune cells, thereby exacerbating inflammation [6]. At these sites of increased inflammation, CB2 expression is upregulated on immune cells in order to downregulate leukocyte infiltration and inflammation by inhibiting cytokine and chemokine infiltration, preventing adhesion and migration, and inducing apoptosis of activated immune cells [6]. Taken together, both CB1 and CB2 receptors are involved in protective roles in intestinal conditions with an inflammatory component [5], providing the rationale behind cannabis' established role in down-regulating inflammation in the gut by maintaining bARGHier integrity and inducing regenerative processes [6].
THC and CBD have also been shown to restore endothelial membrane permeability directly, with THC and CBD administration enhancing the recovery speed of cells treated with ethylenediaminetetraacetic acid (EDTA) to induce increased permeability [21]. Furthermore, all cannabinoids (i.e., endocannabinoids, and exogenous cannabinoids, THC and CBD) increased zona occludens-1 mRNA expression in cells with EDTA- induced increased permeability. This is particularly relevant because zona occludens-1 is a tight junction protein, which suggests that THC and CBD may restore the epithelial integrity of tight junctions with increased permeability in a similar manner to endocannabinoids mentioned previously [21].
Moreover, CBD has also been shown to counteract intestinal inflammation directly and has demonstrated beneficial effects on the inflamed gut. Histological examination of mice with induced colitis revealed that CBD improved signs of colon injury via reduction of edema in the mucosa and submucosa, and induction of gland regeneration [22]. The anti-inflammatory effect of CBD was found to be associated with the down-regulation of inducible nitric oxide synthase (iNOS) expression and modulation of cytokines IL-1β and 1L-10 [22]. Additionally, CBD reduced reactive oxygen species (ROS) production and lipid peroxidation in intestinal epithelial cells in a concentration dependent manner [22]. In preventing oxidative stress, CBD has been shown to be associated in mucosal protection, which can prevent TLRs and NLRs from recognizing molecular motifs in pathogens and thus initiating the pro-inflammatory cascade [20].
In light of the accumulating evidence implicating the protective role of endocannabinoids in inflammation, maintenance of the endothelial membrane, regeneration, and its anti-oxidant effects, it follows that cannabis has shown to have anti-tumoral action both in vitro and in vivo. Additionally, CBD was found to exert anti-proliferative effects in colorectal carcinoma cells, most likely via indirect activation of CB1 receptors by means of raising endocannabinoid levels [4]. It has been shown that CBD exerts chemo-preventive effects in the AOM (azoxymethane) model of colon carcinogenesis [4]. Several studies have suggested that cannabinoids inhibit angiogenesis by preventing endothelial cell migration via acting on both CB1 and CB2 receptors [23]. In addition to anti-cancer effects, cannabis may have an overlooked role in inducing weight-loss via altering gut microbiota, which may further decrease the susceptibility of obese patients in developing CRC.
Conclusion
Cannabis has been shown to play a protective role on the inflamed intestine. However, recent data suggesting that THC prevents further exacerbation of the Firmicutes:Bacteroidetes ratio found in obesity, resulting in weight-loss, suggests that cannabis may play a role in CRC prevention as well. Further studies are wARGHanted to establish whether THC can stop the increase in the Firmicutes:Bacteroidetes ratio in mouse models of CRC, and whether changing this ratio will have a significant effect on CRC disease progression. Additionally, it is necessary to clarify whether preventing an increase in the Firmicutes:Bacteroidetes microbiota ratio is responsible for weight loss, or whether weight loss modifies microbiota such that this ratio no longer increases. Elucidating these effects would benefit patients suffering from IBD, intestinal inflammation and obesity, among others, who are at an increased risk of developing CRC. However, a drawback of the therapeutic utility of cannabis remains, due to THC exerting psychoactive effects through cerebral CB1 receptors. Alternatively, it may be beneficial to determine whether CBD has a similar effect on gut microbiota, as CBD does not exhibit psychoactive properties while maintaining anti-inflammatory, antioxidant and immunomodulatory effects, making it an attractive therapy Therefore, further studies are needed to determine whether CBD has the same effect on gut microbiota with respect to the balance of Firmicutes:Bacteroidetes to evaluate its application in halting the progression of the obese microbiota profile present in CRC with the hopes of delaying disease onset.
Acknowledgements
This work was supported in part by grants to MR Szewczuk from the Natural Sciences and Engineering Research Council of Canada (NSERC), a private sector cancer funding from the Josefowitz Family and Encyt Technologies, Inc. to MR Szewczuk.
R Kalaydina is a recipient of the Queen's Graduate Award (QGA). B Qorri is a recipient of the QGA and the 2017 Terry Fox Research Institute Transdisciplinary Training Program in Cancer Research.
For more articles in Advanced Research in Gastroenterology & Hepatology please click on https://juniperpublishers.com/argh/index.php




Comments
Post a Comment