Updating on Neuroendocrine Tumors New Clinical Evidence in the Search of the Ideal Treatment Sequence_Juniper Publishers
Authored by
Juan Manuel O'Connor
Introduction
Neuroendocrine tumors (NETs) represent a
heterogeneous clinical entity with an increasing incidence as shown by
the different registers available. As opposed to other solid tumors,
NETs represent a challenge, not only from the diagnostic standpoint but
also in terms of treatment strategies. According to the data published
by our Working group, ARGENTUM GROUP [1], with more than 600 patients assessed, roughly 60% of the cases present with metastatic disease from the onset.
The reason why the incidence of advanced disease is
high since early onset may be due to a bias, for most of the physicians
in our Group are oncologists. Moreover, about 20% of the patients
included present poorly differentiated NETs, a heterogeneous subgroup,
with high risk and poor outcome according to the different series
reported.
G grading, in the classification of neuroendocrine
tumors has been proposed by ENETs to identify three categories according
to the proliferative value, Ki 67: Ki 67 under2 %, G1; Ki 67 between 3
and 20%, G2; and wth a Ki 67 over 20%, G3.
Although for therapeutic reasons patients with well
differentiated tumors were classified in the same manner, at present the
cut off point for G3 has been modified. As reported by Sorbye et al. [2]
55% is the parameter to better discriminate this group of patients,
based on a retrospective series including more than 300 patients with
poorly differentiated neuroendocrine tumors. Moreover, the same study
showed that Ki 67 was a predictive factor of response for platinum-based
chemotherapy.
As recently reported and commented by Dr. G. Rindi at
the 2016 Annual ENETs Conference a different biological behavior has
been described even for the so called NEC G3 tumors, that is, poorly
differentiated neuroendocrine carcinomas; a sub classification has been
proposed for NET G3 to better differentiate them from the real NEC G3,
considering that the former present more favorable characteristics for a
good outcome, and even the potential somatostatin receptor expression
in some cases. This sub classification is important because it favors
new treatment options at least in some G3 subgroups, although a
retrospective validation is required for better staging.
In a retrospective series including 136 patients with G2 gastroenteropancreatic tumors, Milione et al, [3]
the Italian group, showed a possible staging system based on
morphological, proliferation features, defects in the mismatch repair
system (dMMR) , CD117 expression and site of origin.
The multivariate analysis in that study showed that
the factors mentioned above were independent in NEC G. Three prognostic
categories were defined based on these factors in G3, a category
including well differentiated tumors vs poorly differentiated tumors and
Ki 67, <55% versus >55%, with a 43.6 month survival in patients
with well differentiated tumors and Ki 67 between 20 and 55%.
In the case of poorly differentiated tumors and Ki 67
ranging between 20 and 55%, median survival was 24.5 months, and
finally the group with the poorest prognosis, poorly differentiated
tumors with a Ki 67 over 55%, with a survival of 5.3 months. This study
clearly shows an active area for clinical research in this subgroup of
patients with limited treatment options at present.
New clinical evidence, randomized studies
At the Annual Meeting of the European Conference held
in Vienna in 2015 the results of different clinical trials in
neuroendocrine tumors were presented. All of them were randomized
comparative studies, evaluating efficacy and tolerance of different
treatment options. Although already available in the field of
neuroendocrine tumors the confirmation of a well designed comparative
clinical trial to show the advantages in terms of survival is still
pending.
The RADIANT4 [4]
study included 302 patients and compared the use of everolimus, (10mg
/day) vs placebo in patients with a diagnosis of Grade 1, 2 advanced,
progressive, well-differentiated, non-functional neuroendocrine tumors
of lung or gastrointestinal origin.
The primary endpoint, progression free survival (PFS)
was achieved; everolimus demonstrated a 52% reduction of progression vs
the non-active treatment arm, which translated intomPFS of 11 months vs
3.9 months in favor of everolimus, which was statistically significant.
The most significant fact in the study is the inclusion of
neuroendocrine tumors of bronchial- lung origin, where the role of m-TOR
inhibitors was not clear.
TheNETTER-1trial compared the use of Lu 177 DOTATATE,
(Lutathera) plus Octreotide (30mg) in patients with NETs of
gastrointestinal origin and advanced disease versus Octreotide (60mg)
every 28 days.
The study included 229 patients, and the primary
endpoint, mPFS, was achieved, with an impressive 80% reduction in risk
of disease progression in the Lutathera arm (HR 0.20 IC 95% 0.130.34).
This result translated into an mPFS not achieved yet in the Lutathera
arm, and a mPFS of 8.4 months in the Octreotide arm (60mg every 28
days). The added value of the study is that it is the first clinical
trial to show favorable results of the use of Lu 177, in a randomized
comparative trial.
Another trial, TELESTAR [4],
studied the role of TELOTRISTAT ETIPRATE, which acts by inhibiting
tryptophan hydroxylase, the rate limiting enzyme in the conversion of
tryptophan to serotonin. The primary endpoint of the study was to reduce
symptoms associated with carcinoid syndrome such as diARGHhea in
patients on octreotide (30mg every 28 days) whose symptoms persisted.
The study included 135 patients, randomized to three
arms; in two arms two different doses of the drug were tested, and the
other arm was given placebo (in fact, these patients were on
Octreotide). In both arms treated with Telotristat a 40% symptom
reduction was observed, and the responses was sustained in time. Also,
there was a reduction in the levels of 5HIAA, as a specific marker of
carcinoid syndrome. This opens the door to a new oral treatment option
for functional tumors, with carcinoid syndrome previously managed with
standard doses of Octreotide.
The data in CLARINET [5]
have been updated; the open phase was presented, that is, the
assessment study in terms of efficacy and safety in patients originally
randomized to lanreotide vs placebo in the CLARINET core study. The open
phase included patients with at least stable disease in the original
study after 2 years of treatment, and patients who had received placebo
during theinitialrandomization and evidenced disease progression.
A total of 88 patients were included, 41 previously
treated with lanreotide and 47 in the placebo arm. Thirty nine percent
of the patients included had intestinal tumors, 38% had pancreatic
tumors, and 23 % had tumors in other locations or of unknown origin. As
for safety, patients who continued on active treatment reported less
adverse effects during this open phase of the study as compared to the
initial phase. The group of patients treated with lanreotide, who were
previously included in the placebo arm, evidenced a higher incidence of
diARGHhea during the initial phase; however, no other adverse events
were observed. The median PFS in the CLARINET core study was 32.8
months.
The median PFS in the open phase in the group who had
not received active treatment before (placebo arm) was 14 months. The
results show the antiproliferative effect and the safety profile of
prolonged treatment in patients with intestinal and pancreatic
neuroendocrine tumors, G1-G2 with a Ki as high as 10% with Lanreotide
(120mg every 28 days).
Update on the Treatment Guidelines of NETs, ENETS
(European Society of Neuroendocrine Tumors). New treatment guidelines
for neuroendocrine tumors of gastroenteropancreatic origin have been
published this year. It should be underlined that these Guidelines are
the result of the Consensus Meeting held by the members of the Advisory
Board in Europe, the United States and Latin America (Argentina and
Brazil). Different topics such as location of the primary tumor and
disease stage are discussed at the Consensus sessions. Also, treatment
options and algorithms in liver metastatic disease and high-grade
neuroendocrine tumors are also dealt with.
In the case of neuroendocrine tumors of intestinal, jejunum- ileum [6]
origin, the role of surgery as a curative procedure is underlined, or
the role a large dissection of the mesenteric lymph nodes as a
palliative procedure.
The management of superior mesenteric artery
involvement should be discussed by a multidisciplinary team, although
resection is not always feasible in advanced disease. In terms of
overall survival (OS), the role or potential benefits of surgery for
primary tumors of intestinal origin in asymptomatic patients with liver
metastases is still debatable. The European Society is conducting a
comparative clinical trial in order to find an answer [7].
A cholecystectomy may be performed during surgery to
avoid the risk of complications due to bile duct lithiasis, another
topic without clear supporting evidence, but with the consensus of the
participants. Another important point underscored in the new version of
the Guidelines is prophylaxis against carcinoid crisis with the use of
somatostatin analogs in patients with carcinoid syndrome. The design of
Treatment Guidelines and an Algorithm for patients with neuroendocrine
tumors and liver metastases has been very important, for it is the most
common location for this type of tumors (Figure 1 & 2).
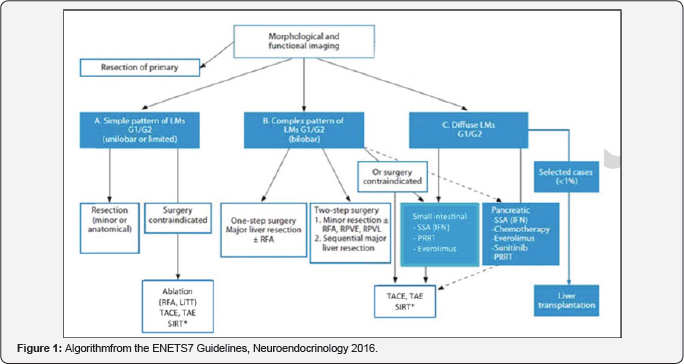
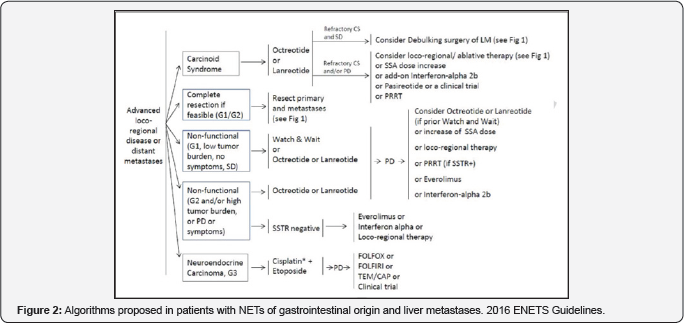
One of the important aspects to underline is the
staging system suggested by ENETS, in relation to 3 subtypes of liver
involvement. In the case of a single pattern, in G1, G2, that is
unilobar or restricted involvement, surgery plays a key role. In the
complex or bilobar type, surgery is the option in some cases, as well as
the liver regional management such as liver embolization of
chemoembolization. Finally, the diffuse type is the most common clinical
scenario, and is the ideal subgroup for different systemic treatment
options or regional liver management, so that surgery is ruled out for
this subset of patients. This subdivision may be easy in the theoretical
setting but in clinical practice it is not so easy to stage patients in
these three categories. Our Group is currently working on a registry of
patients with liver metastases to estimate the frequency of
presentation, in which cases surgery is performed up front, which are
the treatment lines used, according to the preferences of the attending
physicians, etc., in a series of cases in Argentina. Finally, the update
on the treatment Guidelines also includes algorithms for neuroendocrine
tumors with liver metastases, considering some differences between
tumors of gastrointestinal and pancreatic origin.
In the algorithm we should underline four or five
practical questions to be answered in order to define the initial
treatment.First, if the patient presents carcinoid syndrome, i.e. a
functional or non functional tumor; second, is surgery as a curative
procedure an option?; third, if the patient has a functional tumor, and
it is a G1, with a low volume of disease and is asymptomatic; fourth,
the same case as before but with a larger volume of disease and
symptomatic or with progression of disease, the possibility of target
therapy, such as everolimus. Finally, does the patient have a G3 tumor?
In this stetting, the only option isplatinum-based chemotherapy (Figure 3).
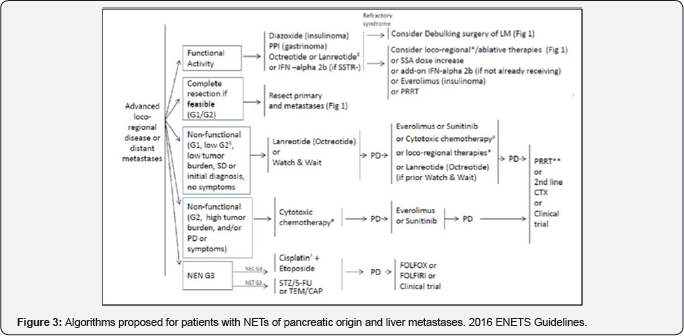
Legend : PD progressive disease, SD stable disease,
SSTR somatostatin receptor, SSA somatostatin analogs, CS carcinoid
syndrome, PRRT peptide receptor radionuclide therapy, LM liver
metastases, TEM/CAP temozolomide/capecitabine, STZ streptozotocin, 5-FU
5-fluorouracil. Similarly, for NETs of pancreatic origin treatment
decisions are based on the same questions presented for (Figure 2) in reference to NETs of gastrointestinal origin.
The subdivision of G3 tumors into G3 NETs, well
differentiated tumors, and G3 NECs, poorly differentiated tumors with
only one treatment option based on platinum- etoposide chemotherapy
should be noted. Furthermore, we should underline that observation is
less feasible in pancreatic tumors, that is, no active treatment in the
setting of advanced disease after the new evidence available from the
CLARINET trial, which included gastrointestinal and pancreatic tumors
with a Ki as high as 10%. Also, there may be a more importantrole for
chemotherapy although with poor levels of evidence to define the best
chemotherapy scheme in this context.
Conclusion
Interesting development is ongoing in the field of
neuroendocrine tumors by cooperative groups and well designed clinical
trials to answer questions about systemic, surgical or local liver
ablative management. Working groups like NANETS, the American Group, or
ENETS, the European Neuroendocrine Tumor Society, have pioneered the
development of both diagnosis and treatment guidelines for this
condition. ARGENTUM, the working group in our country, more humbly and
with certain limitations, studies the local data and provides a chance
for sharing experiences between our region (LATAM) with the leading
centers in the world. Our great challenge for the future is to learn
more about the best treatment sequence for this condition as well as the
molecular characterization of different tumor subtypes, in order to
optimize resources and take a step closer to precision medicine or
personalized medicine in this field. A multidisciplinary approach
together with the integration of the participating specialists, not only
oncologists and surgeons, is the key to succeed and advance in research
to offer patients with this condition the best treatment option.
To Know More About Advanced Research in Gastroenterology &
Hepatology Journal
click on:
https://juniperpublishers.com/argh/index.php
https://juniperpublishers.com/argh/index.php




Comments
Post a Comment