Peptic Ulcer Disease with Gastrointestinal Bleeding in Father and Son with Primary Polycythemia Vera_Juniper Publishers
ADVANCED RESEARCH IN GASTROENTEROLOGY & HEPATOLOGY JUNIPER PUBLISHERS
Authored byJohn Ryan
Keywords
Keywords: PPV: Primary Polycythemia Vera; GIB: Gastrointestinal Bleeding; MPD: Myeloproliferative Disease; VWF: Von Willebrand Factor
Case Report
Case 1
This is a 32 year old male of Asian descent with a known history of Primary Polycythemia Vera (PPV) with a positive JAK 2 mutation. He receives phlebotomy on a regular basis to maintain his hemoglobin below 14mg/dl. He presented with hematemesis and melena. He denied any known risk factors for upper GI bleeding. On presentation his systolic blood pressure was stable but he had a tachycardia with a heart rate 110120. His hemoglobin had descended from baseline of 14 down to 6.6mg/dl. He was administered a proton pump inhibitor (PPI) infusion. Following adequate volume resuscitation, an urgent Esophagogastroduodenoscopywas performed. Within the duodenal bulb, on the posterior wall, a large ulcer with an active bleeding vessel was identified. Hemostasis was achieved using an epinephrine injection and hemoclips. Post procedure, he was continued on the PPI infusion for another 72 hours. He also received one unit of pack red blood cells. He had no further bleeding during his hospital course. His H. Pylori studies were negative. A serum gastrin level was normal.
Case 2
This is a 69 year old male, who is the father of case 1. He also has history of PPV with a positive JAK 2 mutation. He requires phlebotomy every other week to maintain his hemoglobin below 14mg/gl. His most recent phlebotomy was on the day prior to presentation. His initial complaint was of a syncopal episode. While in the emergency department, he developed hematemesis. He did report using two doses of Motrin 800mg within 72 hours of admission.He remained hemodynamically stable and was started on a PPI infusion. His hemoglobin decreased significantly (7.6mg/dl), and he did require a blood transfusion.
Following appropriate resuscitation, an EGD was performed which revealed two duodenal ulcers. The first ulcer was clean based and the second one had a dark red spot. There were no other high risk stigmata and no hemostatic intervention was undertaken. His H. pyloristudies were negative.
The following is a discussion of the pathophysiological mechanisms that predispose subjects with PPV to gastrointestinal bleeding.
Primary Polycythemia Vera and Gastrointestinal bleeding
Epidemiology: Myeloproliferative disease refers to different clonal hematological disorders. These entities include Primary Polycythemia Vera, Essential Thrombocythemia, Primary Myelofibrosis, Chronic Myeloid Leukemia and Unclassifiable Chronic Myeloproliferative Disease [1,2]. PPV is characterized by pan-hyperplastic, neoplastic bone mARGHow with an absolute increase in the red cell mass. The current diagnosis of PPV is based on the World Health Organization's major and minor criteria [2]. The major criteria include Hemoglobin> 18.5 in male (>16.5 in female) and the presence of a JAK 2 Mutation. The minor criteria include endogenous erythroid colony growth, low serum erythropoietin levels, and proliferation of all three lines in the bone mARGHow [2]. JAK2 mutations are highly sensitive and specific for differentiating PPV from other causes of increased red cell mass [3]. A JAK 2 mutation with a concurrent low serum erythropoietin level rules out any other etiology for increase serum hematocrit level [4]. JAK2 mutations result in the loss of inhibition of JH2 pseudokinase [5]. This ultimately leads to hypersensitivity of the mutated stem cell hematopoietic growth factors, and thus a high hematocrit [5].
There are approximately 148,000 cases of PPV in United States with estimated prevalence of 22-57/100,000 [6,7]. Patient with PPV are at higher risk for both thrombotic and hemorrhagic diathesis [8,9]. Hemorrhagic diathesis is rare and are usually mucocutaneous [8,9]. The mucocutaneous nature of the bleeding is suggestive of primary defect in hemostasis [10]. It is difficult to ascertain the exact prevalence of gastrointestinal bleeding (GIB) in subjects with PPV due to the retrospective nature of studies that address this issue. Papadakis et al summarized the complications of myeloproliferative disease from four different studies. Of these, two reported major hemorrhagic diathesis [11]. Bleeding as an initial manifestation of PPV has been reported in3-8.1% of an involved cohort [11]. There are other reports with a significantly higher incidence of hemorrhage in PPV [12,13]. In the ECLAP trial, the rate of total and major bleeding were 2.9% and 0.8% per year [14]. Additionally, 4% of deaths were attributed to bleeding [14]. GI bleeding was the most common cause of major bleeding and the second most common cause of minor bleeding [15]. Major hemorrhagic events requiring blood transfusion and hospitalization were mostly gastrointestinal in origin [8].
Pathophysiology: Gastrointestinal bleeding tendency in PPV is considered to be multifactorial [10]. Use of non-steroid anti-inflammatory (NSAID) agents, age above 60 year, platelet dysfunction, severe thrombocythemia, longer duration of PPV and previous history of GI bleeding have been considered to be the main risk factors in the development of gastrointestinal bleeding in subjects with PPV [8]. Regular or sporadic use of NSAID's increases the risk of gastrointestinal bleeding in PPV significantly by causing ulcers in the gastrointestinal tract and inhibition of the platelet function [16,17].The overall incidence of major GIB in a PPV cohort is 0-0.3% per year, but increase to 0.31.2% per year with concurrent aspirin use [17], Higher aspirin dosage has been associated with a higher risk of GI bleeding. Aspirin use was first studied in the Polycythemia Study Group. 163 PPV patients were randomly assigned to radiophosphorus or phlebotomy, antiplatelet therapy and dipyridamole. This study was stopped due to the higher incidence of GI bleeding in the antiplatelet therapy arm [18]. The data from ECLAP study (which reported lower GI bleeding incidence) should be viewed with caution as this study excluded subjects with previous thrombotic or hemorrhagic events. However, the overall risk of GI bleeding was slightly increased with a relative risk of 1.6 [19]. Advanced age beyond 59 years doubled the risk of GIB in PPV [16]. Dysfunctional platelets are considered to be the cornerstone in the development of gastrointestinal bleeding [10]. Platelet dysfunction in PV may be due to: acquired storage pool deficiency, increased platelet activation, decrease adrenergic receptor expression, impaired response to epinephrine, decreased platelet membrane glycoprotein receptor expression, lack of second- wave adrenaline aggregation or an increased adenosine diphosphate aggregation threshold [10,20]. Platelet dysfunction in PPV is also characterized by high shear stress in the microcirculation can lead to to spontaneous activation of platelets, and resulting in von Willebrand factor (vWF) associated transient plugging of microcirculation. This is followed by degradation of these plugs, with subsequent release of exhausted, defective platelets into the circulation. Acquired von Willebrand syndrome by increased proteolysis by ADAMTS 13 secondary to thrombocytosis has been reported as a culprit for some bleeding episodes in patients with PV [10,21-23]. Peptic ulcer disease is manifested in higher frequency in PV patient compared to the remainder of the population [24,25]. PV patients have been found to a have higher incidence of erosions, ulcers, H. Pylori infection, CagA positivity [24]. Increasedhistamine release has been touted as a potential risk factor for the peptic ulcer disease [24]. PV patients have been proven to have higher serum and urinary histamine level [26]. Increased histamine release has been associated with increases in gastric acid secretion, mucosal blood flow and mucosal permeability, hence predisposing to peptic ulcer disease [27].
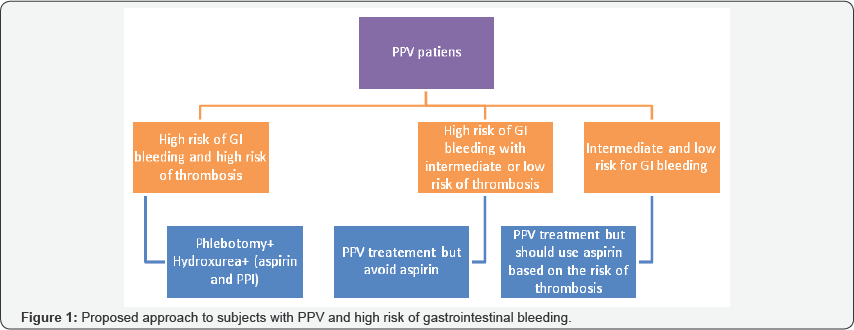
Approach to subjects with PPV: Hence both the thrombotic and hemorrhagic manifestations of myeloproliferative disorders depend on the platelet count. The typical pattern of GI bleeding (whether upper or lower GI bleeding) has not been well reported in literature. There is little data on the best diagnostic approach to subjects with GI bleeding. The GI hemorrhagic diathesis is more evident in the setting of extreme thrombocythemia [28]. The majority of subjects with PPV show a balding pattern suggestive of platelet dysfunction and VWF abnormality. Von Genderen et al reported 100 cases of bleeding in subjects with thrombocythemia, half of which were episodes of GI hemorrhage [28]. Approximately 80% of GI bleeding was from the upper GI tract. In a retrospective study of thrombocythemia, out of 33 subjects with systemic manifestations of the disease, 24% showed evidence of peptic ulcer disease [29]. Torgano et al performed endoscopy in patients with PPV and demonstrated that 74% had either erosions or ulcers on EGD [24]. Egli et al reported a 40% bleeding incidence in their cohort, mainly in the GI tract [30]. The decision regarding whether to use to use aspirin in the PPV population should be individualized based on individual risks as shown in the Figure 1 [8]. Landolfi et al divided the risk associated with GI bleeding into major and minor risk factors. The major risk factors are a platelet count above 1.5, 000,000x109 /ml and a previous history of major bleeding. The minor risk factors are a disease duration of more than 15 years, a platelet count greater than 1,000,000 x 109/ml, and a history of minor bleeding. Subjects are considered high risk if they cARGHy one or more major risk factors, or 3 minor risk factors. The risk of bleeding should generally be weighed against the risk of thrombosis, and treatment with aspirin should be individualized. Those having an age above 60 years and/or a history of thrombosis would be stratified as high risk group for thrombotic events [31]. If there is no contraindication, then this group should be treated with apsirin to decrease the risk of thrombotic events. In high risk patient with a history of GI bleeding, concurrent PPI use should be considered to decrease the risk of upper GI bleeding. The use of aspirin wARGHants caution in subjects with extreme thrombocytosis and/or a history of abnormal bleeding. Such indivuals should be evaluated for platelet dysfunction prior toconsideration of aspirin therapy by obtaining a ristocetin cofactor assay. Aspirin should be avoided if the assay shows less than 30% activity [31,32].
Conclusion
PPV is primarily associated with thrombotic complications, however, gastrointestinal bleeding has been reported. Peptic ulcer disease is frequent and can be multifactorial in origin. If aspirin is considered in these patient with thrombocythemia, von Willebrand factor levels should be checked prior to initiation of antiplatelet therapy. Avoiding NSAID's, Aspirin and concurrent use of PPI may decrease the risk of gastrointestinal bleeding, but would not eliminate it.
For more articles in Advanced Research in Gastroenterology & Hepatology please click on https://juniperpublishers.com/argh/index.php




Comments
Post a Comment