Immunohistochemical Evidence of Lactoferrin in Malignant Gastrointestinal Tumors': A Mini Review
Authored by G Tuccari
Abstract
Lactoferrin (LF), an iron-binding glycoprotein, is
well known to have different physiological activities in humans; in
normal conditions, it has been found in milk, blood, urine as well as in
many external and mucosal secretions. We report herein a mini-review
regarding the LF immunohistochemical pattern in gastrointestinal
malignant tumours obtained from different human districts in order to
acquire a possible explanation for its presence and function.
Keywords: Immunohistochemistry; Lactoferrin; Gastrintestinal; Cancer; HumansIntroduction
Lactoferrin (LF) is an 80kDa glycosylated single chain protein, constituted of ca. 700 amino acids, with high homology among
species, present in milk and colostrums as well as in many body
fluids, such as blood plasma, amniotic fluid, tears, saliva, semen, bile, urine [1,2]. Several functions have been attributed to LF, although the corresponding mechanisms remain still controversial [3];
it appears involved in the regulation of iron homeostasis and
absorption in the bowel as well as in the antimicrobial activity against
bacteria, viruses, fungi and parasites [4]. Moreover, immunomodulatory and anti-inflammatory effects of LF have been reported [3,4]. Finally, LF appears to show some enzymatic properties such as protease, DNAase, RNAase and ATPase [5].
Recently, it has been suggested that LF is involved
in the regulation of some important processes, such as the cycle and the
death of cells, fighting against the carcinogenesis and the development
of metastases [6].
In particular, it has been hypothesized that LF inhibits cell
proliferation and suppresses tumour growth, blocking the transition from
G1 to S in the cell cycle of malignant cells, both in vitro and in vivo [7,8].
Therefore, to better understand the LF anticarcinogenic activity, we
report herein an immunohistochemical analysis of LF in human neoplasms
of different gastrointestinal district in the attempt to elucidate also
its possible pathogenetic role.
Material and Methods
We have reviewed the immunohistochemical pattern of
LF distribution in 101 surgical samples obtained from a corresponding
number of malignant tumours developed in the stomach (30), colon (39)
and gall bladder (32). The study was conducted in accordance with Good
Clinical Practice guidelines and the Declaration of Helsinki and
approved by local ethics committee.
All samples have been fixed in 10% neutral formalin
for 24hrs at room temperature (RT) and then embedded in paraffin at 56
°C. Moreover, the bone/cartilage specimens have been decalcified using
formic acid 5% or EDTA 5%, pH 7.4, for a period not longer than 48hrs.
Depending on the size of mineralised samples. From each block of
malignant neoplastic tissue, 4|im-thick sections were stained with
haematoxylin/eosin for the microscopic evaluation, but parallel sections
were cut and mounted on silane-coated glasses, then dewaxed in xylene
and rehydrated in graded ethanols. Antigen retrieval was performed
before adding primary antibody by heating slides placed in 0.01 M
citrate buffer, pH 6.0, in a microwave oven for three cycles x 5min.
For the immunohistochemical study, sections were treated in a moist chamber at room temperature:
- with 0.1% H2O2 in methanol to block the intrinsic peroxidase activity (30min);
- with normal sheep serum to prevent unspecific adherence of serum proteins (30min);
- with the monoclonal primary antibody against antihuman LF (clone 1A1; Biodesign International, Saco, ME; w.d. 1: 75; 60min);
- with sheep anti-mouse immunoglobulin antiserum (Behring Institute; w.d. 1: 25;30min);
- with mouse anti-horseradish peroxidase-antiperoxidase complexes (Dako Cytomation, w.d. 1: 25; 30min).
For the demonstration of peroxidase activity, the
sections were incubated in darkness for 10 min with 3-3'
diaminobenzidine tetra hydrochloride (Sigma Chemical Co., St Louis, MO),
in the amount of 100 mg in 200 ml 0.03% hydrogen peroxide in phosphate-
buffered saline (PBS). The nuclear counterstain was performed by
Mayer's haemalum. Renal tubular structures within normal kidney samples
as well as portions of parotid gland were utilised as additional
positive controls, as elsewhere suggested [7,8].
The LF immunoreactivity demonstrated in granules of polymorphonuclear
neutrophils was utilised as positive control. Finally, in order to test
the inter-run variability of LF staining, the same LF-positive parotid
sample was utilised in every run. To test the specificity of LF
immunostaining in order to deny the possibility of non-specific
reaction, serial sections of each neoplastic specimen were tested by
replacing the specific antiserum by either PBS, normal rabbit serum or
absorbing with excess of purified human LF from human liver and spleen
(Sigma Chemical Co.) as well as with pre-absorbed primary antibody: the
results obtained were negative.
Immunostained sections were estimated by light
microscopy using an x20 and x40 objective lens and x10 eyepiece; the
assessment of LF immunostained sections was performed on a consensus
basis by two pathologists using a double-headed microscope.
Results and Discussion
All neoplastic samples, routinely stained by
haematoxylin and eosin, exhibited a good morphology, confirming the
histopatological diagnosis; however, parallel sections were adequately
stained by LF immunohistochemistry, with an immunoreactivity generally
localized in the cytoplasm but occasionally in the nucleus.
The mucous neck cells of the antrum and body of the
stomach were positive for LF; moreover, an evident LF immunoreactivity
was encountered in intestinal type carcinomas, whereas diffuse type ones
were always unstained. A clear intense cytoplasmatic immunopositivity
for LF was found in well and moderately differentiated colo-rectal
adenocarcinomas as well as mucinous carcinomas, even if some
undifferentiated cases were unreactive; the LF immunostaining was also
encountered in neoplastic elements present in metastatic lymphnodes,
when the primary cancer was stained.
In gallbladder, a positive LF immunoreactivity was
found in a variable share of adenocarcinomas, mainly represented in
papillary or glandular areas, while sarcomatoid, squamous or mucinous
components were negative; the number of immunostained elements as well
as the staining intensity showed some differences in the context of the
same tumour. (Figure 1)
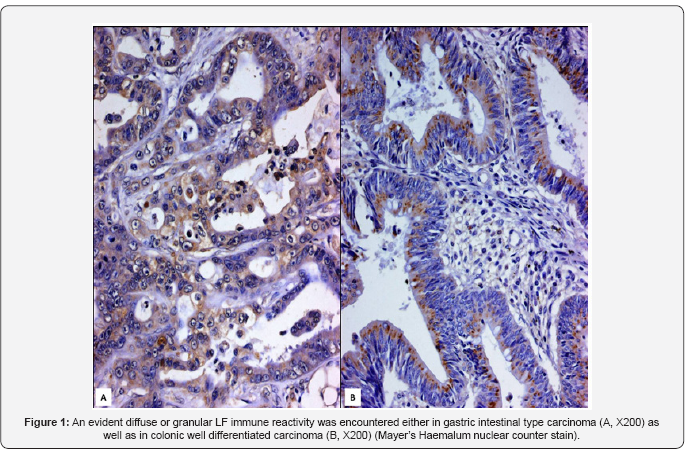
In the present study, we have analyzed LF
immunoexpression in a series of human gastrointestinal malignant
tumours, showing that LF presence was not exclusively localized to the
cytoplasm, but also in the nucleus. However, the site of LF
immunolocalization in both the nucleus and cytoplasm has not been
considered surprising since this glycoprotein has been thought to be
involved in ribosomal biogenesis and after its transport into the
nucleus; LF is able to bind specific DNA sequences, thus activating
transcription [9].
Moreover, we have shown that heterogeneity in LF immunoexpression
between different malignancies as well as inside the same tumour were
not infrequent; if this observation could reflect different cell
subpopulations, the stage in the cell cycle or instead some metabolic
abnormalities should be verified by methods other than morphological
analysis [10-19].
The origin of LF in human malignant tumours has not
yet been fully elucidated. It is well known that LF has a high affinity
for iron, which has been considered an essential nutrient for cells that
are dividing rapidly such as tumour cells, taking part in various
metabolic processes such as oxydative phosphorylation and RNA and DNA
synthesis [1,5].
Therefore, neoplastic elements should be able to produce LF in order to
make a greater amount of iron available for their turnover, similarly
to that elsewhere suggested [7,20].
Alternatively, the localization of LF in malignant cells may not
reflect an intracellular synthesis, reflecting instead the degree of
transmembranous iron transfer as the consequence of defective or
functionally impaired LF-receptors, already documented on the surface of
target cells as well as in human neoplastic cell lines [21].
In our casuistry, the LF immunostaining was never
founded in relation to the site, grade and stage of malignant tumours,
excluding thus its role as predictive or prognostic neoplastic markers.
Nevertheless, the immunohistochemical evidence of LF was largely
confined to differentiated carcinomatous histotypes, such as
differentiated glandular carcinomas of the stomach, colon and
gallbladder, while anaplastic and undifferentiated carcinomas were
always unstained; consequently, it may be suggested a role for LF as
marker of glandular or acinar differentiation, similarly to that already
pointed out in other malignancies [20,22,23].
Conclusion
The protective effects of LF have been demonstrated on chemically induced tumors of rodents [1];
moreover, it has been previously reported that LF is able to inhibit
the development of experimental metastases in mice, mainly by an
increase of NK cells and T lymphocytes expressing CD8, CD4 and IFNy [24].
Meanwhile, other potential mechanisms have been suggested regarding the
role of LF in the process of human carcinogenesis, including induction
of programmed cell death, prevention of angiogenesis and regulation of
cell cycle protein expression [25,26].
In fact, LF is able to trigger the apoptotic process by the activation
of caspases 3 and 8 as well as the FAS signaling pathway [24]; on the other hand, LF was also shown to inhibit tumour-initiated angiogenesis in vitro and in vivo, possibly by blocking endothelial function and inducing IL-18 production [25]. Moreover, it has been reported that LF promoted growth ARGHest either at the G1 to S transition in breast cancer cells [8] as well as at the G0-G1 checkpoint in oral and neck cancer cells [27];
finally, LF demonstrated its ability to regulate cell growth by
controlling the level of retinoblastoma protein, a key tumour suppressor
involved in cell cycle progression [28].
Nevertheless, whatever was the mechanism ofaction of LF in tumours; we
probably still require additional investigations about the opportunity
for new applications of LF in cancer, mainly regarding its nutraceutical
function as well as its ability to potentiate chemotherapy.
To Know More About Advanced Research in Gastroenterology &
Hepatology Journal
click on:
https://juniperpublishers.com/argh/index.php
https://juniperpublishers.com/argh/index.php




Comments
Post a Comment