Weight Reduction Improves Adipokines Profile and Glucose Control of Patients with Nonalcoholic Fatty Liver Disease
Authored by Shehab Mahmoud Abd El-Kader
Background: Non-alcoholic fatty
liver disease (NAFLD) is a common health problem that usually associated
with insulin resistance and obesity. The prevalence of NAFLD is about
15–20% of the general Asian population and affecting up to 30% of the
population worldwide. At present, treatment options are limited and
pharmacological management of NAFLD has had disappointing results.
Lifestyle interventions (diet and exercise) are the standard treatment
of NAFLD.
Objective: As the available
previous studies involving the effects of weight loss on adipokines
profile and glucose control of nonalcoholic fatty liver disease patients
is limited in number; this study aims to measure response of
adiopocytokines and glucose control response to weight reduction in
patients with nonalcoholic fatty liver disease
Methods: One hundred male patients
with NAFLD were included into this study and divided into two equal
groups. Group (A) received aerobic exercise training in addition to diet
regimen. Group (B) received no treatment intervention.
Results: There was a 27.48%,
21.59%, 30.49%, 33.72% & 10.67 % reduction in mean values of leptin,
resistin, insulin, HOMA-IR & BMI respectively in addition to 35.69%
& 29.27% increase in the mean values of adiponectin & QUICKI
respectively in group (A) at the end of the study. The mean values of
leptin, resistin, insulin, HOMA-IR & BMI were significantly
decreased in addition to significant increase in the mean values of
adiponectin & QUICKI of group (A) received aerobic exercise training
in addition to diet regimen. While the results of group (B) received no
treatment intervention were not significant. In addition, there were
significant differences between mean levels of the investigated
parameters of group (A) and group (B) after treatment (P<0.05).
Conclusion: Based on our findings, a
10 % reduction in BMI is effective to improve glucose control and
adipokines dysregulation in patients with non-alcoholic fatty liver.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most
common liver disease worldwide [1]. It is comprised of a spectrum of
disorders characterized by liver steatosis with > 5% of hepatocytes
infiltrated with fat in individuals with no history of alcohol abuse
(< 30 g/d in men and < 20 g/d in women) and no competing
etiologies for hepatic steatosis [2,3]. The presentation of the disease
ranges from what can be considered as “silent liver disease”, or fatty
steatosis, to non-alcoholic steatohepatitis (NASH) [4]. Approximately
10%-25% of patients with silent liver disease develop NASH, and 5%-8% of
those will develop liver cirrhosis within 5 years [2,5]. Furthermore,
12.8% of patients with liver cirrhosis will develop hepatocellular
carcinoma (HCC) within 3 years [6].
Nonalcoholic fatty liver disease (NAFLD) is regarded
as the hepatic component of metabolic or insulin resistance (IR)
syndrome, increasing recently in parallel with the epidemics of obesity
and type 2 diabetes mellitus (T2DM) [7,8]. NAFLD is now a global public
health problem, with a prevalence of 10–46% in the general United States
(US) population and of 6–35% in the rest of the world [9]. It is the
most common cause of chronic liver disease in US adults. NAFLD ranges
from nonalcoholic
simple steatosis (SS) to nonalcoholic steatohepatitis (NASH)
characterized by steatosis, inflammation and/or fibrosis [9].
However, IR and adipokines contribute to the pathogenesis of SS
and the progression to NASH and NASH related cirrhosis [10].
NAFLD is also associated with an increased risk for developing
cardiovascular disease, insulin resistance (IR), chronic kidney
disease, post-operative complications after major liver surgery
and colorectal cancer [11-13].
Obesity, especially visceral obesity, is frequently associated
with NAFLD and their coexistence in the same individual
increases the likelihood of having more advanced forms of liver
disease [14]. NAFLD occurs in 60%-95% of people with obesity
[15]. However, in cases of NAFLD associated with obesity, serum
levels of leptin are increased [16]. Unlike other adipokines, serum
levels of adiponectin are decreased in obesity and its associated
medical complications [17]. Compared with healthy controls,
adiponectin levels are lower by more than 50% in NASH patients
[18]. Adiponectin expression is decreased by 20%-40% during
the development of NAFLD, from simple steatosis to NASH [19].
In addition, Resistin is a hormone secreted from adipocytes that
has a positive relationship with body composition characteristics
and insulin resistance [20]. Increased insulin resistance follows
the increase of resistin. However, its exact mechanisms are not
known yet [21].
Visceral adipose tissue (VAT) is also a source of a number
of secreted adipocyte-derived cytokines called adipokines [22].
The most well described adipokines are adiponectin, an insulin
sensitizer, and leptin, a hormone mainly secreted by adipocytes
[23], which play functional roles in NAFLD pathogenesis. Obesity
is considered a state of central and peripheral leptin resistance,
and obese individuals, as well as individuals with NAFLD and
NASH, have higher circulating levels of leptin [24].
Recent studies use diet, physical activity and behavior
modification to help promote weight loss in NAFLD patients
[25]. Several studies have shown that weight loss is successful
in improving liver enzymes, insulin sensitivity, reducing
inflammation and liver histology [26-28]. A randomized
controlled trial conducted by Promrat et al. [29] used a
combination of diet, physical activity and behavior modification
to trigger 7%-10% weight loss in obese NASH patients. Those
who achieved a minimum of 7% weight loss had improvements
in their liver histology.
The purpose of this study was to investigate the impact of
weight reduction on glucose control and adipokines in type-2
diabetic patients with NAFLD.
Material and Methods
Subjects
One hundred male patients with NAFLD with body mass
index (BMI) ranged from 30 to 35 Kg/m2, their age ranged from 35 to 55 years. Participants were included in this randomized
controlled study and divided into two equal groups; group (A)
received physical training combined with dietary measures. The
second group (B) received no intervention and considered as a
control group. Participants were identified from a large number
of patients attending the Liver Clinic in King Abdulaziz University
Teaching Hospital, with a histological diagnosis of NAFLD. The
diagnosis of NAFLD was based on the following criteria:
- Elevated aminotransferases alanine and/or aminotransferase (AST and/or ALT)
- Liver biopsy showing steatosis in at least 10% of hepatocytes; and
- Appropriate exclusion of liver disease of other aetiology including alcohol- or drug-induced liver disease, autoimmune or viral hepatitis, cholestatic or metabolic/ genetic liver disease. These other liver diseases were excluded using specific clinical, biochemical, serologic tests radiographic and/or histological criteria.
Exclusion criteria included smoking; hypertension,
personal history of cardiovascular diseases, thyroid disease and
orthopedic problems inhibiting treadmill training. This study
was approved by the Scientific Research Ethical Committee,
Faculty of Applied Sciences, King Abdulaziz University. Informed
consent was obtained from all participants. All participants
were free to withdraw from the study at any time. If any adverse
effects had occurred, the experiment would have been stopped,
with this being announced to the Human Subjects Review Board.
Evaluated parameters
Measurement of glucose control and adiopkines markers
serum level:After a 10 hours overnight fast, venous blood
samples were drawn to determine levels of leptin, adiponectin
and resistin. Serum level of leptin was measured with DRG leptin
ELISA Catalog number EIA-2395, supplied by DRG instruments
GmbH, Germany and serum level of adiponectin was determined
using AviBion human adiponectin (Acrp 30) ELISA kit ref. no.
ADIPO 25 (Orgenium Laboratories, Finland), while serum level
of resistin was measured by ELISA using commercially available
kits (resistin: Rapidbio, West Hills, CA, USA; CK-18: PEVIVA,
Alexis, Grunwald, Germany) according to the manufacturer’s
instructions. Human insulin was measured with an insulin
kit (Roche Diagnostics, Indianapolis, IN, USA) using a cobas
immunoassay analyzer (Roche Diagnostics). Insulin resistance
was assessed by homeostasis model assessment (HOMA-IR).
HOMA-IR = [fasting blood glucose (mmol/l) _ fasting insulin
(mIU/ml)]/22.5 [30]. However, insulin sensitivity was assessed
by The quantitative insulin-sensitivity check index (QUICKI)
using the formula: QUICKI=1/ [log(insulin) + log(glucose)] [31].
All serum samples were analyzed in duplicates.
Body mass index (BMI):Body weight of participants in both
groups was measured (HC4211, South Korea) while wearing hospital gowns and undergarments. Where the height was
measured using digital stadiometer (JENIX DS 102, Dongsang).
Body Mass Index (BMI) was computed as BMI= Body weight/
Height2. The international standard definition of obesity was
used. Patients were classified as underweight (BMI ≤ 18.5),
normal (18.5 ≤ BMI ≤ 25), overweight (25 ≤ BMI ≤ 30), or obese
(BMI ≥ 30).
Procedures
The physical training program:The aerobic treadmillbased
training program (PRECOR 9.1/ 9.2, China) was set to
65%- 75% of the maximum heart rate (HRmax) according to a
modified Bruce protocol. This rate was defined as the training
heart rate (THR). After an initial, 5-minute warm-up phase
performed on the treadmill at a low load, each endurance training
session lasted 30 minutes and ended with 5-minute recovery and
relaxation phase. All patients performed three weekly sessions
(i.e. 36 sessions per patient over a 3-month period).
The prescribed low calorie diet:The interview-based food
survey was performed for all patients by dieticians to specify
previous food habits and possible anomalies in dietary behavior.
The prescribed low calorie diet was balanced, with 15% as
protein, 30 to 35% as fat and 50 to 55% as carbohydrate, on
average, in order to provide about 1200 Kilocalories daily for
two months for whole participants in this study.
Statistical Analysis
The mean values of leptin, adiponectin, resistin, HOMA-IR,
QUICKI and BMI obtained before and after three months in both
groups were compared using paired “t” test. Independent “t” test
was used for the comparison between the two groups (P<0.05).
Results
Study population’s characteristics
One hundred patients with NAFLD were enrolled including
53 women and 47 men, their age ranged from 35 to 55 years.
Participants were included in this randomized controlled study
and divided into two equal groups; group (A) received physical
training combined with dietary measures, while group (B)
received no intervention and considered as a control group.
The two groups were considered homogeneous regarding the
demographic and clinical variables (Table 1).
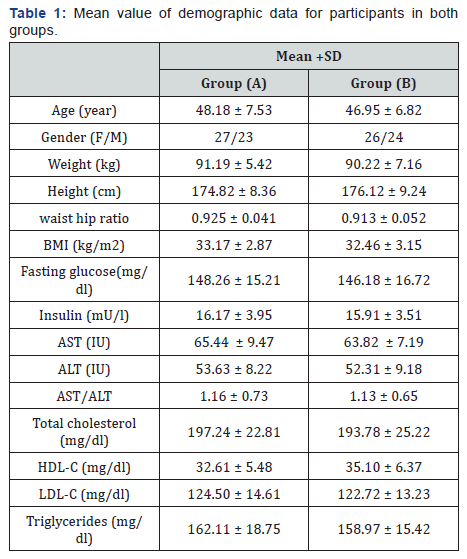
BMI: Body mass index; AST: Aspartate aminotransferase; ALT:
alanine aminotransferase; AST/ALT: Aspartate aminotransferase
/alanine aminotransferase ratio; HDL-c: High density lipoprotein
cholesterol; LDL-c: Low density lipoprotein cholesterol.
There was a 27.48%, 21.59%, 30.49%, 33.72% & 10.67 %
reduction in mean values of leptin, resistin, insulin, HOMA-IR &
BMI respectively in addition to 35.69% & 29.27% increase in the
mean values of adiponectin & QUICKI respectively in group (A) at
the end of the study. The mean values of leptin, resistin, insulin,
HOMA-IR & BMI were significantly decreased in addition to
significant increase in the mean values of adiponectin & QUICKI
of group (A) received aerobic exercise training in addition to diet
regimen. While the results of group (B) received no treatment
intervention were not significant. Also, there were significant
differences between mean levels of the investigated parameters
in group (A) and group (B) after treatment (Table 2-4) (P<0.05).
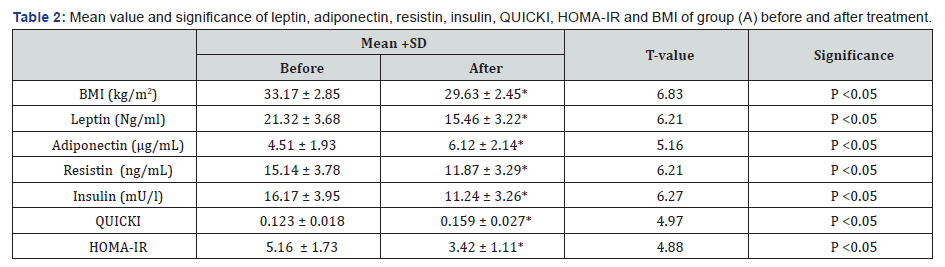
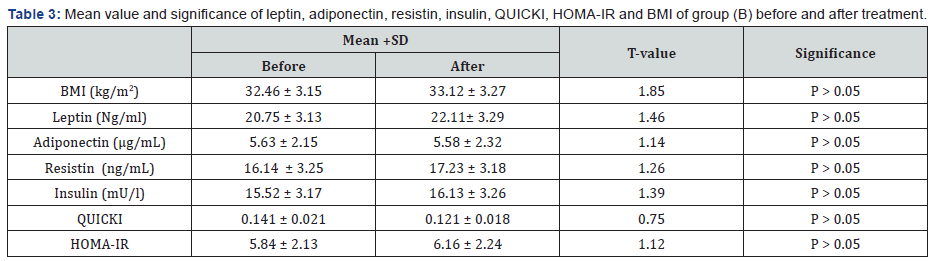
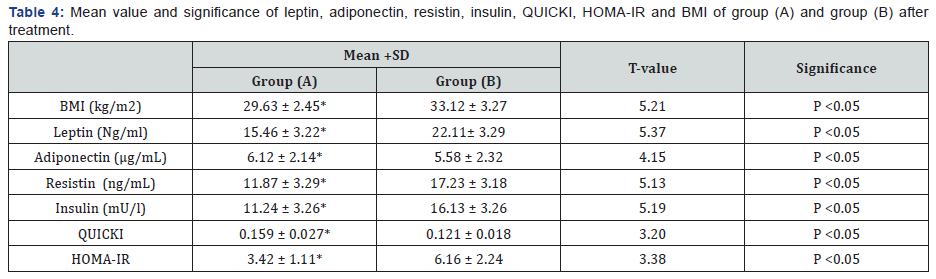
BMI: Body mass index; HOMA-IR: Homeostasis Model
Assessment-Insulin Resistance (HOMA-IR) index; QUICKI: The
quantitative insulin-sensitivity check index; (*) indicates a
significant difference between the two groups, P < 0.05.
BMI: Body mass index; HOMA-IR: Homeostasis Model
Assessment-Insulin Resistance (HOMA-IR) index; QUICKI: The
quantitative insulin-sensitivity check index.
BMI: Body mass index; HOMA-IR: Homeostasis Model
Assessment-Insulin Resistance (HOMA-IR) index; QUICKI: The
quantitative insulin-sensitivity check index; (*) indicates a
significant difference between the two groups, P < 0.05.
Discussion
Non-alcoholic fatty liver disease (NAFLD) is an obesityassociated
disease [32]. The prevalence of obesity and insulin
resistance (IR) is increasing worldwide: over 78 million
Americans are obese, and one-third have high IR [33]. It is
now recognized that insulin resistance in obesity is largely
consequential to adipose tissue inflammation and adipokine
dysregulation [34]. To date, weight loss is the only confirmed
therapy for the treatment of NAFLD, and lifestyle interventions
remain the cornerstone of management [35,36]. The results of
this study proved that weight reduction significantly modulate
the insulin resistance and adipokines dysregulation among
patients with NAFLD, these results are in line with many previous
studies.
Regarding glucose control, this study proved that life style
modification (aerobic exercise and diet regimen) significantly improved insulin resistance because of weight reduction.
These results agreed with Ryan et al. [37] demonstrated in an
insulin-resistant population with NAFLD a reduction of liver
steatosis and an improvement of insulin sensitivity after 6 week
of the Mediterranean diet, compared to current dietary advice.
Moreover, Kontogianni et al. [38] reported that higher adherence
to the Mediterranean diet was not associated with a less
likelihood of having NAFLD, but it was associated with a lower
degree of insulin resistance and less severe liver disease among
patients with NAFLD. Moreover, Angelico et al. [39] proved
that 5%-10% weight loss as a result of diet regimen modulates
insulin resistance in patients with metabolic syndrome. In
the other hand, Hallsworth et al. [40] showed that 8 weeks of
resistance exercise in sedentary adults with NAFLD resulted in
a an improvement in insulin resistance, also Bacchi et al. [41]
conducted a randomized controlled trial of 31 sedentary adults
with type 2 diabetes and NAFLD comparing the effects of 4
months of aerobic and resistance training on insulin sensitivity
and hepatic steatosis. Hepatic fat content, hepatic steatosis and
insulin sensitivity were reduced in both intervention groups.
Several mechanisms have been proposed to be responsible for
the increases in insulin sensitivity after exercise training. These
include increased post-receptor insulin signaling, increased
glucose transporter protein and mRNA, increased activity of
glycogen syntheses and hexokinase, decreased release and
increased clearance of free fatty acids, increased muscle glucose
delivery and changes in muscle composition [42].
Concerning the levels of adipokines, this study proved
that weight loss because of 12 weeks of life style modification(aerobic exercise and diet regimen) significantly increased the
level of adiponectin and reduced the levels of both leptin and
resistin. These results agreed with Copaci et al. [43] reported
that 86overweight persons who achieved significant reductions
in body weight through 12 months of physical activity and
low caloric diet recognized significant modifications in insulin
resistance, leptin and adiponectin. Oh et al. [44] stated that
12-week exercise training program remarkably increased the
serum adiponectin level and equivalent improvement of insulin
resistance. mentioned that data obtained from 72 obese, middleaged
men with NAFLD who completed a 3-month program of
exercise and diet regimen that induced weight loss resulted
in increased level of adiponectin and decreased level of leptin
in addition to modulation of insulin resistance [45]. The
combination of diet and exercise for three months decreased
circulating leptin and BMI more than diet alone [46]. In another
prospective study, the combination of aerobic and resistance
exercise for 12 weeks decreased leptin levels in NAFLD patients
more than aerobic exercise alone, although BMI was similarly
affected [47]. In a cross-sectional study, fewer NAFLD patients
were engaged in resistance training compared to controls,
whereas the rates of those engaged in aerobic exercise were
similar [48].
Jung et al. [49] proved that long-term exercise program and
a diet led obese individuals to reduce significantly the level
of resistin and leptin. Jones et al. [50] have studied the effect
of 8-week aerobic exercise on lipid levels of serum, leptin,
adiponectine, resistin, peptide YY, and ghreline in overweight
adolescents and reported a significant decrease of resistin.
Kadoglou et al. [51] studied the effect of 16-week regular aerobic
exercises with a VO2max of 50 to 85 percent on resistin levels in
patients with type 2 diabetic and overweight ones. They reported
a significant decrease of this hormone among the participants. In
the study by Elloumi et al. [52] two months of exercise with the
weight loss, led to a significant decrease in resistin level among
obese adolescents. Balducci et al. [53] reported that 12 months
of regular physical activity could decrease the level of resistin in
patients with diabetes and obesity. Rashidlamir et al. [54] stated
that aerobic training for 8 weeks, 4 sessions in a week, and with
the intensity of 70-80 % of maximum heart rate resulted in
significant reduction in BMI, fat percentage and serum resistin
levels in young females.
Conclusion
Based on our findings, a 10 % reduction in BMI is effective
to improve glucose control and adipokines dysregulation in
patients with non-alcoholic fatty liver.
To Know More About Advanced Research in Gastroenterology &
Hepatology Journal
click on:
https://juniperpublishers.com/argh/index.php
https://juniperpublishers.com/argh/index.php




Comments
Post a Comment