Cytomegalovirus causing Haemorrhagic Colitis Requiring Hemicolectomy in a Kidney Transplant Recipient
Abstract
71 year old lady with medical history of end stage
renal disease due to hypertension received a living related kidney
transplant. She received thymoglobulin induction at dose of 6mg/kg and
her maintainence immunosuppression consisted of tacrolimus, prednisone
and mycophenolic acid. She received valganciclovir for 3 months for CMV
prophylaxis as both donor and recipient were positive for serum CMV IgG
antibodies. She presented with melena and fevers 4 months post
transplant. She developed hematochezia and went into hemorrhagic shock
immediately after presentation and required multiple transfusions.
Bleeding scitinography showed active bleeding in the region of the
distal small bowel. Coiled embolization failed to control the bleeding
and she underwent emergent right sided hemicolectomy. Her serum CMV
virus result was 3,900,000 copies/ml. She was treated with
valganciclovir and mycophenolic acid was stopped. Pathology report of
the resected segment of colon showed severe ulceration of entire colon
with cells containing viral cytopathic effects, confirmed by
immunohistochemistry, as CMV.
Keywords: Kidney; Cytomegalovirus
Introduction
Cytomegalovirus (CMV) is one of the most common viral
infections in post-transplant immunocompromised patients. It can
present in a wide variety of ways including colitis, gastritis,
hepatitis and leucopenia. We present a novel case of severe hemorrhagic
colitis due to CMV infection.
Discussion
What is CMV
Cytomegalovirus is a common herpes virus [1]. CMV is
the most common and single most important viral infection in solid organ
transplant recipients. CMV infection usually develops during the first
few months after transplantation and is associated with clinical
infectious disease (e.g, fever, pneumonia, GI ulcers, hepatitis) and
acute and/or chronic graft injury and dysfunction. Many people do not
know they have it, because they may have no symptoms. But the virus,
which remains dormant in the body, can cause complications during
pregnancy and for people with a weakened immune system. Also known as
HCMV, CMV, or Human Herpes virus 5 (HHV-5), cytomegalovirus is the virus
most commonly transmitted to a developing fetus.
Transmission
The virus can spread in a number of ways [2]:.
Touching your eyes or the inside of your nose or mouth
after coming into contact with the body fluids of an infected person.
This is the most common way CMV is spread because it’s absorbed through
the mucous membranes.
Through sexual contact with an infected person.
Through the breast milk of an infected mother [3].
Through organ transplantation or blood transfusions.
Through the placenta, from an infected mother to her unborn child, or during birth [4].
Pathogenesis
CMV has the potential to spread in the bloodstream to
all organs, but only produces overt disease if the viral load increases
to high levels. This is normally prevented by a robust immune response,
so that the infected individual usually remains asymptomatic. However,
this benefit comes at the cost of committing more and more immunological
resources
to controlling CMV with time, so that the overall function of the
immune system is impaired [5].
The CMV genome is composed of lineal, double-stranded
DNA, surrounded by a protein lining, called matrix, which
contains phosphoproteins (pp65, pp150, etc.) that are highly
immunogenic and capable of deregulating the cellular cycle of
the host cell. This lining is surrounded by glycoproteins (gB, gN,
gO, gH, gM, gL) necessary for the virus infectivity: entrance to the
host cell, cell-to-cell dissemination, and maturing.
The fusion of the virus with the cell is mediated by the viral
glycoprotein gB. The fusion is followed by the entrance of the
nucleocapsid and the lining proteins to the host cell cytoplasm;
the nuclei are translocated rapidly and pp65 antigen, a marker
of infection, is detected in the serum in less than an hour. The
main reservoirs of CMV are the fibroblasts, myeloid cells,
and endothelial cells. The infection of endothelial cells and
macrophages plays an important role in the latency, and this
seems to be a critical point in the maintenance of CMV in the
host.
The start of the replication takes about 12-24 hours after the
infection of the cell, and the cytopathic effect in the viral culture
could be seen after 7-14 days. As with other herpes viruses, CMV
invades the host cell, inhibits protein synthesis, and liberates
viral DNA to the nuclei, where the replication starts immediately.
A strategy that it shares with other herpes viruses is the ability
of stopping the immune response of the host by inhibiting RNA
formation, blocking the presentation of antigenic peptides of
the cell surface, and blocking apoptosis. These mechanisms
prompt to a latent infection that may be reactivated in transplant
recipients
Immunologic mechanism of rejection
The immune recognition of foreign antigens in the graft is
mediated by MHC class I and II. Class I molecules are expressed
in all nucleated cells and platelets, while class II are expressed
by B lymphocytes, cells of the monocyte-macrophage system and
dendritic cells. The T-cells and non lymphoid cells show class II
proteins only when they are activated by cytokines. Rejection
depends on the coordinated activation of T-cells and antigenpresenting
cells [6].
For example, in kidney rejection, tubulitis is one of the major
diagnostic criteria and consists of the invasion of the tubular
epithelium by lymphoid cells. The CD8 lymphocytes are the
cells mainly involved in tubulitis development. These cells are
attracted by the β-chemokine secretion, especially, MCP-1 and
MCP-1β (monocyte chemotactic peptides). Also participating
are macrophage inflammatory protein MIP-1α and RANTES
(activation regulated peptides expressed in T-cells and possibly
secreted).
Something similar occurs in heart transplantation. The
expression of self antigens to avoid being recognized and
damaged is a constant mechanism.
Treatment
There’s no cure for CMV, and treatment for the virus generally
isn’t necessary or recommended for healthy children and adults.
Newborns and people with compromised immune systems
i.e. Patients after kidney transplantation, however, need
treatment when they’re having symptoms of CMV infection, such
as pneumonia. The kind of treatment depends on the symptoms
and their severity.
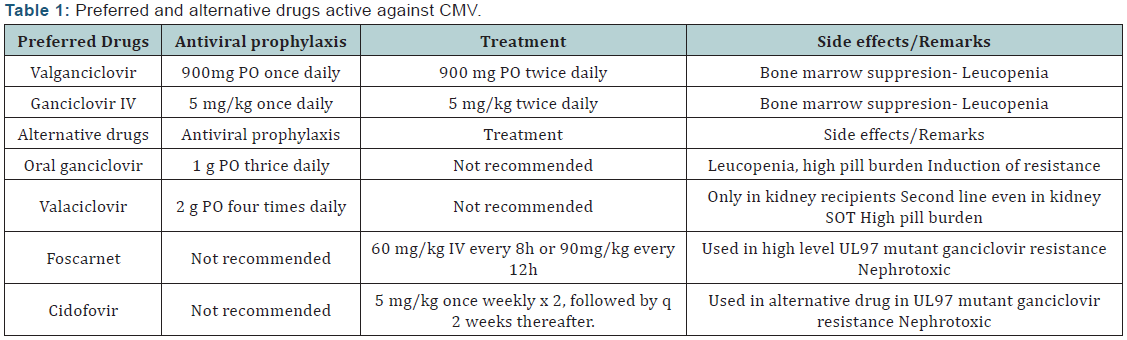
Treatment of CMV in solid organ transplant [7] recipients
reduces the risk of allograft injury and death [. The two main drugs
used for treating CMV disease are intravenous (IV) ganciclovir
(5-mg/kg every 12 hours) and oral valganciclovir (900-mg twice
daily). Oral valganciclovir achieves comparable blood levels to
IV ganciclovir and is recommended for the treatment of mild to
moderate CMV disease in solid organ transplant recipients.
In a study of 321 adult solid organ transplant recipients
with CMV disease, the clinical and virologic outcomes were not
significantly different between those patients who received oral
valganciclovir or IV ganciclovir. The rate of viremia eradication for
valganciclovir group and IV ganciclovir group were comparable
- 45.1% versus 48.4% at day 21, and 67.1% versus 70.1% at day
49, respectively. The median time of viremia eradication (21
versus 19 days), side effect profiles, and treatment outcomes
were also comparable between the two groups .
IV ganciclovir is preferred drug for treatment of severe or
life-threatening CMV disease or in those with questionable
gastrointestinal absorption. IV ganciclovir is also recommended
for those with very high viral load. Oral ganciclovir should never
be used in the treatment of CMV disease because of poor oralbioavailability
leading to sub-therapeutic blood levels.
In addition to the antiviral therapy, it is strongly emphasized
that a cautious reduction in immunosuppression will help
in the clearance of infection. CMV occurs as a result of an
over-immunocompromised state, hence, the reduction in
immunosuppression will allow for the recovery or the generation
of CMV-specific immunity that will allow longer-lasting control
of the virus infection.
The duration of antiviral therapy should be
individualized
and be guided by resolution of clinical symptoms and viral load
monitoring. Viral load kinetics that have shown to help predict
clinical response to antiviral therapy include a lower pretreatment
viral load, a faster rate of viral load decline in response
to therapy, and viral suppression at the end of treatment. In a
recent study which used the WHO international standard for
reporting, patients with a pretreatment CMV DNA < 18,200 IU/
mL were more likely to have CMV disease resolution. Moreover CMV
suppression < 137 IU/mL was predictive of clinical response to
therapy (Table 1).
Prevention
Careful hygiene is the best prevention against CMV. Health
care workers have the greatest chances of exposure, but because
of precautions used in the health care setting, their risk of
acquiring the disease is low.
CMV in solid organ transplantation (SOT)
While most infections in immunocompetent individuals
are benign and self-limited, CMV is an important cause of
morbidity and mortality in individuals with underdeveloped or
compromised immune function, including transplant recipients.
Despite significant advances in its diagnosis and therapy, CMV
continues to have a major impact on patient and allograft survival
among solid organ transplant (SOT) recipients through a variety
of direct and indirect effects.
Advances in the field of CMV and solid organ transplantation
will be facilitated by the development of [8] optimized threshold
for viral diagnosis, [9] effective vaccines for prevention, [10]
diagnostic assays to stratify risk of late onset CMV disease by
immunological monitoring, and newer antiviral agents with
unique mechanisms of action and ideally with much less toxicity.
Conclusion
Gastrointestinal CMV disease is an increasingly recognized
clinical problem in immunocompromised patients [11]. Its
presentation can be very mild diARGHhea, nausea and vomiting
which is common, to very severe colitis which is rare. Our patient
developed hemorrhagic shock due to severe colitis and ultimately
required hemicolectomy [11a-d]. If kidney transplant recipient
presents with a gastrointestinal bleed, CMV disease should be considered immediately. Timely diagnosis and treatment is
extremely important as it can have fatal consequences.



Comments
Post a Comment