Association between Adipocytokines, Systemic Inflammation and Oxidative Stress Biomarkers among Obese Type 2 Diabetic Patients_Juniper Publishers
ADVANCED RESEARCH IN GASTROENTEROLOGY & HEPATOLOGY JUNIPER PUBLISHERS
Authored by Essam H. Jiffri
Abstract
Background: Type 2 diabetes mellitus (T2DM) is usually associated with microvascular and macrovascular complications because of abnormal level of inflammatory cytokines, adiopcytokines and oxidative stress biomarkers.
Objective: The aim of this study was to measure the degree of association between adipocytokines, systemic inflammation and oxidative stress biomarkers among obese type 2 diabetic patients. Material and Methods: One hundred non-smokers obese type 2 diabetic patients were included in this study, the mean age was 46.39±5.42 year and mean body mass index was 32.48±3.26 kg/m2.In the other hand another one hundred non-diabetic subjects not suffering of any disease, were participated in the study as a control group, the mean age was 47.88±4.71 year and mean body mass index was 26.52±2.17 kg/m2.
Results: There were significant elevations in tumor necrosis factor- alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), leptin, malondialdehyde (MDA) levels and significant decreases in adiponectin, GPX, GSH and SOD levels were detected among obese T2DM subject in addition, IL-6, TNF-α, CRP and leptin showed a strong direct relationship with MDA and a strong inverse relationship with glutathione (GSH), (GPx) and superoxide dismutase (SOD). While, adiponectin showed an inverse relationship with IL-6, TNF-α, CRP & leptin and a strong inverse relationship with MDA (P<0.05).
Conclusion: The present study added more confirmations to closely link obesity with adipocytokines, systemic inflammation and enhanced oxidative stress biomarkers in obese T2DM.
Keywords: Adipocytokines; Cytokines; Obesity; Oxidative Stress; Type 2 Diabetes
Introduction
Type 2 diabetes mellitus (T2DM) accounts for more than 90% ofthose with diabetes [1]. According to World Health Organization (WHO), estimate that about 366 million people will have diabetes by year 2030 worldwide [2]. Risk cardiovascular disorders is 2-4 times more among T2DM than non-diabetic subjects [3] where about 80% of T2DM patientshave cardiovascular complications asperipheral arterial disease, stroke and heart disease [4]. Diabetes is a group of metabolic diseases that leads to impaired wound healing [5], higher susceptibility to infections [6] atherosclerosis [7], micro-and macrovascular alterations [8] even with optimal glycemic control [9], however poor diabetic control is usually associated with many co-morbidities due to systemic inflammation in T2DM patients [10-12].
Obesity is a state of increase mass of adipose tissue that is considered as active endocrine organ [13] which secretes inflammatory cytokines and adipocytokines that are involved in systemic and local regulation of several metabolic processes [14]. However, disturbed adipose tissue endocrine function in obesity is associated chronic low-grade systemic inflammation and facilitate development of obesity comorbidities as T2DM and vascular disorders [15].
Adipose tissue is the main source of cytokines as increased body fat mass is usually associated with high level of inflammatory cytokines such as C-reactive protein (CRP), tumor necrosis factor- alpha (TNF-α) and interleukin-6 (IL-6) [16,17].While, lymphocytes and macrophages produce low level of anti-inflammatory interleukin-10 (IL-10) in T2DM patients [18] produces low-grade systemic inflammation [19] which play an important role in the pathogenesis of cardiovascular disorders [20-22] and insulin resistance [23-26].
Adiponectin is an adipocytokine that is inversely correlated to the degree of obesity [27,28], which improve insulin sensitivity, and has an anti-inflammatory, antioxidant and anti-hypertensive effects [29-31] in addition to prevention of endothelial dysfunction [32]. In the other hand, leptin correlated to the degree of obesity [33]. Leptin is involved in food intake and energy expenditure modulation; however, obesity is associated with hyperleptinemia that means increased resistance to leptin that is associated with insulin resistance, hypertension and pro- atherogenic condition [33,34].
Oxidative stress considered the principle cause of micro- and macrovascular alterations among T2DM [35] due to excessive production of reactive oxygen species than the antioxidant capacity of the cells [36]. Several mechanisms can contribute to the systemic hyper-inflammatory status in diabetes, including dyslipidemia that modulates the function and activity of myeloid cells, and the increase of oxidative metabolism with enhanced production of reactive oxygen species (ROS) [37]. As a defense mechanism cells produce antioxidants (AOs) that prevent or limit oxidative tissue injury as overproduction of ROS may result in an imbalance between ROS and AOS leading to oxidative stress and tissue damage [38] by several mechanisms, including damage of DNA, lipid membranes peroxidation, enzymes oxidation and stimulation of pro-inflammatory cytokines [39].
The aim of this study was to measure the degree of association between adipocytokines, systemic inflammation and oxidative stress biomarkers among obese type 2 diabetic patients.
Methods and Materials
Subjects
One hundred obese T2DM patients visiting king Abdulaziz University Hospital, Jeddah, Saudi Arabia, were included in this study, the mean age was 46.39±5.42 year and mean body mass index was 32.48±3.26 kg/m2. Initially, a physician at King Abdulaziz University Hospital examined all participants; their medical history was taken to collect information about general condition, physical activity and current medications if any All subjects with any cardiovascular conditions (those with a known history of uncontrolled hypertension, congenital and rheumatic heart diseases), any pulmonary disease (obstructive or restrictive lung diseases), were excluded from the study. In the other hand one hundred non-diabetic subjects not suffering of any disease, were participated in the study as a control group, the mean age was 47.88±4.71 year and mean body mass index was 26.52±2.17kg/m2. The Ethics Committee of the Faculty of Applied Medical Sciences, King Abdulaziz University, approved this study. All participants signed a written informed consent.
Measurements
In all subjects, clinical and anthropometric data were collected at the time of enrollment. Independent assessors who were blinded to group assignment and not involved in the routine treatment of the patients performed clinical evaluations and laboratory analysis. Body mass index (BMI) was calculated on the basis of weight (kilograms) and height (meters), and subjects were classified as normal weight (BMI 18.5-24.9kg/ m2), overweight (BMI 2 5-29.9kg/m2), and obese (BMI≥30kg/ m2). In addition, between 07:30 and 09:00, after an overnight fast of 12h fasting blood sample was drawn. Triglycerides, high- density lipoprotein cholesterol (HDL-c) and plasma glucose concentration and insulin were determined (Roche Diagnostics GmbH, Mannheim, Germany) using commercially available assay kits. Insulin resistance was assessed by homeostasis model assessment (HOMA-IR). HOMA-IR=[fasting blood glucose (mmol/l) fasting insulin (mIU/ml)]/22.5 [40].
- Measurement of oxidative stress markers and antioxidant status: For all participants serum (from 10 ml blood in plain vial) and plasma (from 5ml blood in EDTA vial) were separated from the sample within 30 min of collection and was stored in pyrogen free polypropylene cryo-tubes at (-80 °C) until analysis. Assessment of lipid markers for peroxidation as malondialdehyde (MDA) were determined according to Buege and Aust. [41]. However, Anti-oxidant status, glutathione (GSH) that was determined by the method of Beutler and colleagues [42], in the other hand, glutathione peroxidase (GPx) and superoxide dismutase (SOD) were measured by the method of Nishikimi and colleagues [43].
- Measurement of inflammatory cytokines and adipocytokines:Venous blood samples after a 12-hours fasting were centrifuged at 4 °C (1000 X g for 10min). "Immulite 2000” immune-assay analyzer (Siemens Healthcare Diagnostics, Deerfield, USA) analyzed IL-6 level. However, TNF-α and CRP levels were measured by ELISA kits (R&D, USA) by using ELISA technique (ELX 808; Bio- Tek Instruments, USA).In addition, plasma sample with K2EDTA was collected after centrifugation and stored at-80 °C to analyze leptin and adiponectin with commercial kits (Randox).
Statistical Analysis
Independent "t” test was used to compare the investigated parameters between both groups (P<0.05). However, the degree of correlation between IL-6, TNF-α, CRP, leptin, adiponectin, MDA, GPX, GSH and SOD was calculated with Pearson's correlation coefficients (r).
Results
Detailed baseline characteristics of the patients with T2DM subjects and non-diabetic subjects control presented in Table 1. There was a significant difference for all characteristics of the obese T2DM subjectsversus non-diabetic subjects, except in the age and gender (Table 1).
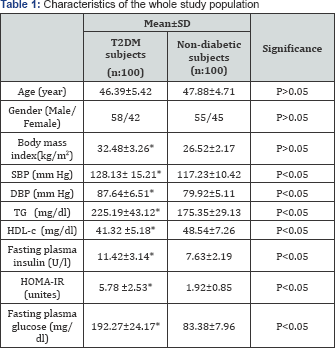
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TG: Triglyceride; HDL-c: High-density lipoprotein cholesterol; HOMA-IR: Homeostasis Model Assessment-Insulin Resistance Index;*Significant level (p<0.05).
However, there were significant elevations in IL-6, TNF-α, CRP, leptin, MDA levels and significant decreases in adiponectin, GPX, GSH and SOD levels were detected among T2DM obese subjects (Table 2).
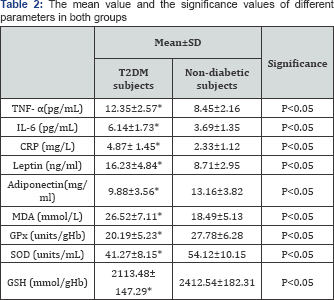
BMI: Body mass index; TNF- α tumor necrosis factor - alpha; IL-6: Interleukin-6; CRP: C- reactive protein; CD: conjugated dienes; MDA: Malondialdehyde; GPx: Glutathione peroxidase; SOD: Superoxide dismutase; GSH: Glutathione; *Significant level (p&0.05).
The IL-6, TNF-α, CRP and leptin showed a strong direct relationship with MDA and a strong inverse relationship with GPX, GSH and SOD. While, adiponectin showed an inverse relationship with IL-6, TNF-α, CRP & leptin and a strong inverse relationship with MDA in obese T2DM subjects (Table 3) (P&0.05).
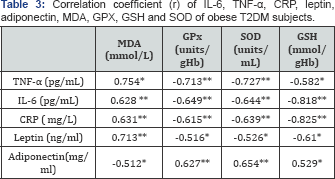
Spearman's correlation was used *: P < 0.05**: P < 0.01
Discussion
Currently, T2DMis the most prevalent metabolic disorder worldwide [44], which is related to vascular problems [45,46]. The main finding of our study was significant elevations in IL- 6, TNF-α, CRP, leptin, MDA levels and significant decreases in adiponectin, GPX, GSH and SOD levels were detected among obese T2DM subjects in addition, IL-6, TNF-α, CRP and leptin showed a strong direct relationship with MDA and a strong inverse relationship with GPX, GSH and SOD. While, adiponectin showed an inverse relationship with IL-6, TNF-α, CRP & leptin and a strong inverse relationship with MDA (P<0.05). Our present investigation added more confirmations to closely link obesity with adipocytokines, systemic inflammation and enhanced oxidative stress biomarkers in T2DM.
Firstly, levels of MDA as a biomarker for oxidative stress were elevated, depletion in GPXand GSH levels along with extreme inhibition of SOD activityin obese T2DM, these results are in line with many previous studies.
Ustundag et al. [47] found an increase in the concentration of MDA in obese group as compared with the control group. In addition, Condoner-Franch et al. [48] showed that, erythrocyte of obese patients had higher MDA and lower GSH concentrations than healthy subjects. However, Al-Menabbawy et al. [49] stated that significant low levels of SOD enzyme was detected among obese adolescents as compared with control group. While, Habib et al. [50] enrolled 72 obese subjects of both sexes and 40 non-obese subjects, their age ranged between 5-17 years , they found significant increased levels of TNF-a, IL-6, leptin, MDA and fasting blood sugar and significant reduction in GSH and SOD activity were detected among obese individuals as compared with control group. Also, Codoner-Franch et al. [51] stated that Nitric oxide (NO) production increased among 60 obese children associated with increased metabolic risk factors included insulin resistance index, serum lipid profile and blood pressure correlated with abdominal obesity, inflammatory markers and oxidative stress markers. Similarly, Hirao et al. [52] confirmed that abdominalobesity is associated with production of increasedreactiveoxygenspecies among 96 Japanese male diabetic patients. Moreover, de Souza Bastos et al. [53] enrolled 100 subjects with diabetes and divided them according to dyslipidemic and diabetic status and they found that diabetics presented significantly higher levels of MDA, IL-6 and TNF-α, however subjects with normal blood glucose level and dyslipidemia presented significantly high levels of IL-6 and TNF-α when compared subjects with normal blood glucose level without dyslipidemia, in addition, presence of DM complications positively correlated with MDA levels.
Excessive adiposity produces oxidative stress via several mechanisms. Firstly peroxisomal and mitochondrial oxidation of fatty acids produce ROS. Secondly, mitochondrial respiratory chain generates free radicals by over-consumption of oxygen associated with mitochondrial oxidative phosphorylation along with generating ROS. By lipid-rich diets that may alter oxygen metabolism in addition to diminished antioxidant enzymes activity [54]. Moreover, obesity is characterized with excessive storage of energy in adipocytes because of energy imbalance which lead to hyperplasia and hypertrophy of adipocytes which in turn induce oxidative stress by adipocytescorrelated inflammatory macrophages. Based on the above, there is a close link among obesity and oxidative stress [55].
Secondly, levels of leptin, TNF-α, IL-6 and CRP were increased and level of adiponectin in obese T2DM subjects in the present study as compared with non-diabetic subjects. Moreover, because adipokines induce the production of ROS generating oxidative stress, we can suggest that adipocytokines elevation is one of the main causes for the obtained high obesity- induced oxidative stress. These obtained results strongly suggest that obese T2DMpatients are more susceptible to persistence of inflammation and to development of obesity-related complications that involve oxidative stress in their pathogenesis especially cardiovascular complications. Our present findings confirm that of Fernandez-Real et al. [56] & Kern et al. [57] who showed that increased IL-6 in obesity increased the risk for cardiovascular complications. Ottobelli et al. [58] proved that levels of adipocytokines included adiponectin and resistin were reduced, markers of inflammation included CRP, IL-6, adenosine deaminase (ADA) and dipeptidyl peptidase-IV (DPP-IV) activities were increased and levels of oxidative stress included MDA and ferric reducing antioxidant power - FRAP were increased among 68 obese subjects. Indulekha and colleagues stated that increased visceral fat level was associated with impaired glucose tolerance, reduced serum adiponectin along with increased TNF-α, CRP, HOMA-IR and visfatin among 49 subjects with impaired glucose tolerance and 93 subjects with T2DM [59]. Upon all, we strongly support the above mentioned mechanisms for excessive oxidative stress induction in obesity. It is established now that free radicals can cause DNA-protein damage. Therefore, we can expect more lipid peroxidation and protein damage including hemoglobin, DNA and protein in the body fluids and tissues of obese T2DM. Hence, the reduction in SOD activity and in GSH level along with persistent elevation in MDA levels in the current study may create oxidant conditions that favor the development of co-morbid diseases sooner.
Conclusion
The present study added more confirmations to closely link T2DM with adipocytokines, systemic inflammation and enhanced oxidative stress biomarkers.
For more articles in Advanced Research in Gastroenterology & Hepatology please click on https://juniperpublishers.com/argh/index.php




Comments
Post a Comment