Hepatocellular Carcinoma and Sorafenib: How can we improve on the Current Standard of Care in Advanced Disease?
Authored by Olowokure Olugbenga
Introduction
Sorafenib at a starting daily dose of 400mg twice
daily (800mg/d) is considered the standard systemic therapy for advanced
unresectable hepatocellular carcinoma (HCC), in patients with
well-preserved liver function. It remains the only FDA-approved
chemotherapeutic agent with a category 1 NCCN recommendation for
patients with advanced liver cancer. Due to complaints regarding side
effects voiced by patients themselves and the reluctance to continue at
full dose by many practicing physicians. We retrospectively reviewed
patients in our liver cancer database that started sorafenib at a total
daily dose of 400 mg (200 mg twice daily). This single institution
retrospective review, evaluated the experience of the impact of starting
sorafenib at a total daily dose of 400mg daily.
Results
A total of 33 patients (M: F, 21: 12) with mean age
of 59.8y (SD: 12.40) were included in the analysis/ met inclusion
criteria, the median duration of follow up after starting treatment was
8.7 months. AFP was elevated (>8.3ng/ml) in 23 (70%). Of those with
abnormal AFP, AFP decrease > 50% from baseline in 8 (35%). Initial
dose tolerability (defined as ability of patient to stay on prescribed
dose of 400 mg/day for at least 1 month without the need for dose-
reduction) was observed in 70% of patients. 49% needed dose reduction
while 58% were able to dose escalate at some point in therapy. Median
duration on 200 mg twice daily dose (400mg/d) was 3 months prior to any
adjustment (CP-A: 3 months, CP-B: 2 months). Mean duration of sorafenib
use was 8.88m (CP-A-10.04 months, CP-B-6.2 months), 26 (79%) patients
had progression and 24 (73%) patients died during follow up. There was
no difference in progression-free survival between CP-A and B. Following
first progression of disease 53% (10/19) evaluable patients continued
sorafenib. OS was 79% at 3 months, 67% at 6 months, 50% at 9 months, and
40% at 12 months. The most common patient reported toxicities were
fatigue 28(88%), diARGHhea 17(53%) and hand/foot skin rash 14(43%) [1].
Discussion
In November 2007 the Food and Drug Administration
(FDA) approved sorafenib based on the results of the SHARP trial, an
international randomized placebo controlled trial in patients with
inoperablehepatocellular carcinoma. This was a groundbreakingadvancement
in the field of HCC. The SHARP trial [2] and Asia-Pacific trials [3]
confirmed the efficacy of sorafenib in advanced HCC. In both of the
trials patients in the treatment group received sorafenib 400 mg twice
daily. In the SHARP trial among the 297 patients that initially received
one dose of sorafenib, 226 discontinued sorafenib. 38% discontinued the
medication secondary to adverse events and 12% withdrew consent. The
median duration of treatment was 5.3 months [2].
80% ofthe patients in the sorafenib group had treatment related adverse
events with 30% reported as grade 3 or higher. Despite using a lower
starting dose, of sorafenib in our patients. The toxicities reported by
the patient population on the SHARP Trial and our the patient population
we reviewed appeared to be different, with a higher percentage of
specific toxicities reported by our patient population. Interestingly,
fatigue and diARGHhea (reported as 39% and 22% on the SHARP trial,) were
found to be much higher in our patient population 88% and 53%. This
seems to be a recurring theme noted among physicians in practice in the
United States and may suggest a difference in the ability to tolerate
higher doses of this medication in majority of the U.S. population as
compared to European and Asian patients.
In an attempt to build on successes of the multi
kinase inhibitor demonstrated in SHARP and Asia Pacific Trials, multiple
phase III studies using a backbone of full-dose Sorafenib in
combination with study agents compared to sorafenib at standard dose
have been conducted. The chart provided below summarizes two of these
major trials (Table 1).
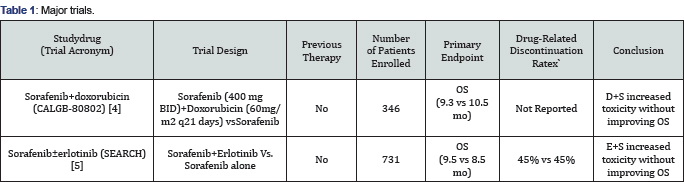
Unfortunately, no such trial to date has resulted in
statistically significant improvements in survival. Multiple mechanisms
are likely responsible for this with one of these possibly due to
unacceptable toxicities once dual agents at individual maximal tolerated
doses are instituted [4,5].
Conclusion
In this single institution
retrospective study of outcomes following initiation of dose-reduced
sorafenib at 200 mg twice daily in advanced HCC. We postulate that this
reduced dose approach did not seem to result in worse outcomes compared
to historical controls possibly due to the ability to tolerate therapy
for a longer duration of time. While one would be remiss in comparing
apples to oranges, a prospective trial would be crucial to understanding
the impact of reduced dose sorafenib on progression free survival and
overall survival in advanced HCC patients. It would be interesting to
prospectively compare a reduced dose of Sorafenib (200 mg twice daily)
in combination with a promising agent to singleagent Sorafenib (400 mg
twice daily) in an attempt to build on and improve progression-free
survival and overall survival in this patient population.
To Know More About Advanced Research in Gastroenterology &
Hepatology Journal
click on:
https://juniperpublishers.com/argh/index.php
https://juniperpublishers.com/argh/index.php




Comments
Post a Comment