All-oral Treatment with Daclatasvir and Asunaprevir for HCV Genotype 1 and Impact of Add-on Therapy with Lipid Modulators-Juniper Publishers
Abstract
Objective: Despite improved
treatment options, many patients with hepatitis C virus (HCV) infection
remain HCV-positive. The all-oral therapy with daclatasvir and
asunaprevir has recently been approved for treatment of patients with
HCV. We conducted a prospective study to investigate the effectiveness
and safety of this therapy.
Methods: We treated 696 patients
with HCV genotype 1 with daclatasvir and asunaprevir for 24 weeks.
Resistance-associated substitutions (RASs) in HCV were analyzed by
direct sequencing of the HCV NS3 and NS5A domains.
Results: HCV RNA declined rapidly
after treatment initiation, and 90.9% of patients achieved a sustained
virological response at 12 weeks after treatment termination (SVR12).
Elevation of serum transaminases and alkaline phosphatase, pyrexia,
headache, malaise, and skin eruption were common adverse events. In
multivariate analysis, treatment history of simeprevir and HCV RASs were
selected as adverse predictive factors, meanwhile addition of
pitavastatin and eicosapentaenoic acid (EPA) was selected as a favorable
predictive factor associated with SVR12.
Conclusion: Treatment response of
HCV was favorable enough during the daclatasvir and asunaprevir therapy.
HCV RASs and prior treatment history with simeprevir could weaken the
efficacy of this therapy, whereas, the lipid modulators, pitavastatin
and EPA, could enhance it through a synergistic antiviral effect.
Keywords: Hepatitis C virus; Eicosapentaenoic acid; Statin; Daclatasvir; Asunaprevir
Introduction
Approximately 170 million people are infected with
hepatitis C virus (HCV) worldwide and natural history studies have shown
that 5%–20% of patients develop cirrhosis after 20 years of infection
[1,2]. People who test positive for HCV antibodies account for 1.4%–1.7%
of the general population in Japan, and a few million individuals are
estimated to have chronic HCV infection. The majority of patients with
HCV in Japan are older than those in the United States and Europe, and
≥60% of the population is aged ≥60 years in Japan [3]. This aging
population is characterized by an increasing incidence of progressive
liver disorders.
Pegylated-interferon (peg-IFN) plus ribavirin (RBV)
combination therapy was the standard of care for chronic hepatitis C
until the 2000s. However, in patients infected with genotype 1 HCV
(HCV-1 patients) with high viral load, at most, 50% of patients achieve a
sustained virological response (SVR) after the therapy [4,5]. Response
to treatment based on
IFN is influenced by virus-related factors including viral load,
genotype, and amino acid substitutions at core 70 and 91; hostrelated
factors, such as sex, age, insulin resistance, disease stage,
response to previous antiviral therapy, and IL28B polymorphism;
as well as therapeutic factors, such as dose and duration of
treatment [6-16].
The direct-acting antiviral agents (DAAs) have made a huge
impact on hepatitis C therapy. The HCV NS3 protease inhibitor
telaprevir (TVR), in combination with peg-IFN and RBV, was
approved for the treatment of serogroup 1 chronic hepatitis C
in September 2011. This combination has achieved SVR rates of
80%–90% for HCV-1 patients [17,18]. The second-generation
protease inhibitors simeprevir (SMV) and vaniprevir were
approved and also showed SVR rates of 80%–90% [19,20]. These
treatments was not sufficient in some cases, and SVR rates for
DAA-based peg-IFN/RBV treatment in patients with intractable
IL28B polymorphism were still 50%–70%, and only 30%–50%
of null responders achieved SVR [21]. Furthermore, these
treatments were associated with severe anemia, skin disorder,
renal dysfunction, pyrexia, general malaise, hematological
abnormalities, and psychiatric symptoms, and many patients
were ineligible for IFN treatment because of age or advanced
liver fibrosis [17-21].
Combination therapy with first-in-class selective NS5A
replication complex inhibitor daclatasvir (DCV; BMS-790052)
and potent NS3 protease inhibitor asunaprevir (ASV; BMS-
650032) was approved for HCV-1 patients with chronic hepatitis
or compensated cirrhosis in July 2013 in Japan. Monotherapy
with each drug is associated with treatment refractoriness
because of the emergence of resistant virus. However, DCV and
ASV have different modes of action and different characteristics
that cause resistance-associated substitutions (RASs) in HCV,
combined use of both drugs is expected to yield a high SVR rate
of at least 80%–90% [22,23]. Although the combination therapy
is also indicated for compensated cirrhosis, a low incidence of
adverse events and high treatment tolerability is expected, which
is explained by the lack of IFN and RBV use [24-29].
HCV life cycle is closely correlated with lipid metabolism in
hepatocytes. It has been suggested that serum cholesterol and
statin use predict virological response to combination therapies
[30]. Recent studies have shown that virological response is
improved by addition of fluvastatin or pitavastatin to peg-IFN/
RBV treatment [31-33]. Moreover, statins were associated with a
reduced risk of hepatocellular carcinoma (HCC) in a large cohort
of patients with diabetes [34]. In other studies, it has been
demonstrated that polyunsaturated fatty acids (PUFAs) inhibit
HCV replication by a mechanism that is independent of their
roles in regulating lipogenesis [35-38]. Recently, we have shown
that addition of pitavastatin and EPA to peg-IFN/RBV treatment
improved SVR rates in HCV-1 patients, especially in those with
the intractable IL28B genotype, and addition of pitavastatin and EPA might activate innate immunity, block viral entry into
hepatocytes, and suppress lipogenesis in the liver [39]. However,
little is known about the additive effect of lipid modulators in
inhibiting HCV replication during DAA treatment.
We conducted a prospective multicenter study to investigate
the efficacy of dual oral therapy with DCV and ASV. We also
present here post-marketing efficacy and safety assessment
of dual oral combination therapy with DCV and ASV in HCV-1
patients. The predictors of treatment efficacy were investigated
and are discussed, including addition of lipid modulators,
pitavastatin and EPA.
Methods
Fukuoka Kanzo Treatment Research Group conducted a
prospective multicenter study to investigate the efficacy and
safety of all-oral treatment with DCV and ASV in HCV-1 patients.
Eligible patients were men and women aged ≥20 years with
genotype 1 chronic hepatitis C or compensated cirrhosis. Patients
were excluded if they had evidence of hepatic decompensation,
suspected HCC or active cancer at entry, pregnancy in progress
or planned during the study period, organic obstruction of
the digestive tract, serious cardiovascular lesions, severe
infection, serious trauma, or severe or unstable complications,
such as other organ failure. From September 2014 to May
2016, 696 patients were enrolled, but the study was restricted
to 649 patients for whom data were available for 12 weeks
post-treatment follow-up. The physician in charge gave the
patients a full explanation of the study objectives and methods,
adverse reactions and risks and countermeasures for them,
and protection of private information. Patients who provided
consent received treatment with DCV 60 mg once daily and ASV
100 mg twice daily. The duration of treatment was 24 weeks, and
patients were followed up for ≥12 weeks after the last treatment
dose. The study was conducted in accordance with the ethical
principles of the Declaration of Helsinki and was approved by
the Ethics Committee of each hospital. Written informed consent
was obtained from all patients before enrollment.
Hematological, biochemical and virological parameters were
determined by the clinical laboratory at each hospital. Serum
HCV RNA concentrations were determined by the COBAS TaqMan
PCR HCV test (Roche Diagnostics, Tokyo, Japan). SVR was defined
as undetectable HCV RNA at week 12 after completion of therapy
(SVR12), and rapid virological response (RVR) was defined as
undetectable HCV RNA at week 4 of treatment. Genotyping for
the IL28B (rs8099917) polymorphisms was performed when
practically possible by TaqMan SNP Genotyping Assays (Applied
Biosystems, Tokyo, Japan) that used polymerase chain-reactionbased
restriction fragment length polymorphism assays. HCV
RASs were analyzed by direct sequencing of the HCV NS3
protease and NS5A domains.
Statistical analysis was performed using JMP version 10.0.0
software (SAS Institute, Cary, NC, USA). Differences between categorical variables were analyzed using Fisher’s exact test or
χ2-test. Mann–Whitney U test was used for continuous variables.
Multivariate analysis was used to identify factors independently
associated with achievement of SVR12. The odds ratio (OR) and
95% confidence intervals were also calculated. P values <0.05
were considered to be statistically significant.
Results
Patient characteristics
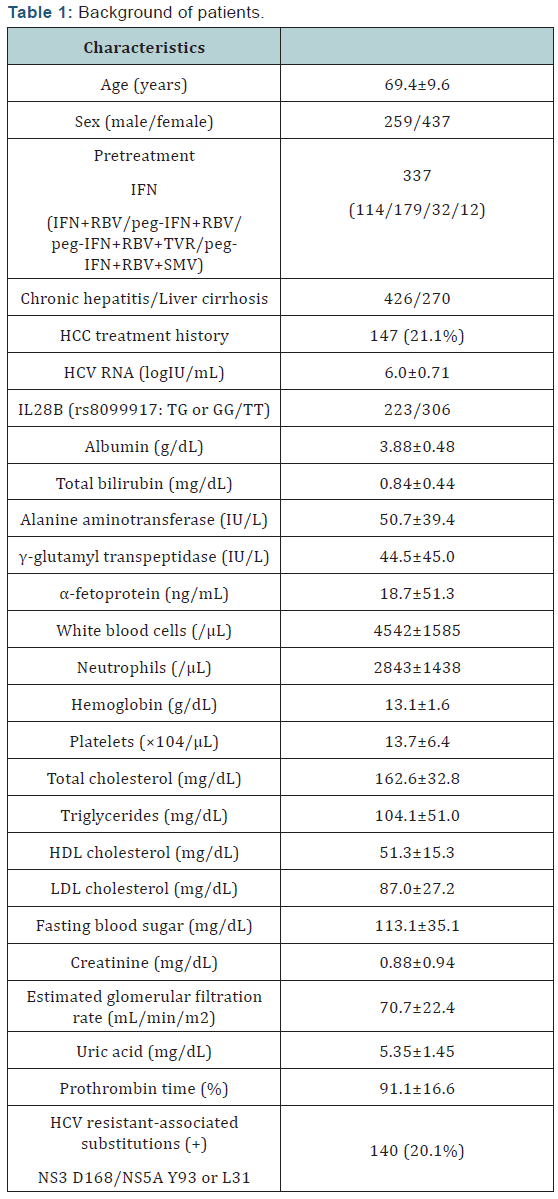
HCV-1 patients with chronic hepatitis or compensated
cirrhosis (n=696) were enrolled and started treatment (Table
1). The enrolled population was older (median 70 years) than
patients with previous IFN-based therapy (early 60s), reflecting
HCV epidemiology in Japan, and predominantly female (63%)
[5,40]. All patients were Asian and 39% had advanced liver
fibrosis (compensated cirrhosis) and 147 (21%) had prior
treatment for HCC. Most of the enrolled patients had genotype 1b
infection (only 2 patients had genotype 1a), consistent with the
high proportion of this subtype in Japan. About 40% of patients
had the intractable IL28B genotype GT/GG (rs8099917), which
was higher than the proportion of individuals with IL28B
genotype in Japan. This might have resulted from the high number
of patients with failure to previous IFN-based treatment. A total
of 359 patients were treatment-naïve and 44 were previously
exposed to DAAs (32 to TVR and 12 to SMV) with peg-IFN and
RBV. Baseline serum albumin and total cholesterol levels, and
platelet count were lower, reflecting the older age and advanced
liver fibrosis in the enrolled population. A total of 140 patients
had HCV with RASs (16 with NS3 D168 and 124 with NS5A Y93
or L31) at baseline.
A total of 649 of the 696 enrolled patients reached follow-up
of 12 weeks after treatment. Sixty-nine patients discontinued the
study treatment: 29 dropped out because of viral breakthrough,
and study drugs were suspended in 40 because of ALT elevation
(n=11), onset of HCC (n=9), general fatigue or illness (n=8),
exacerbation of hepatic encephalopathy (n=3), skin eruption
(n=3), brain infarction (n=1), brain hemorrhage (n=1), and
patient request (n=4). All patients remained in the study for
assessment of SVR.
Virological response
High rates of virological response were obtained at all
time points. HCV RNA declined rapidly and was lower than the
sensitivity limit in 67% of patients after 2 weeks, and 83% of
patients achieved RVR (Figure 1A). Mean reductions of HCV RNA
from baseline at 4 weeks after treatment initiation were –5.8 log
IU/mL, and the response of HCV was not affected by age, sex, liver
fibrosis, or previous treatment response. HCV RNA positivity
at 4 weeks was significantly higher in patients with HCV with
RAS in NS3 D168, NS5A L31, or NS5A Y93, and RVR rate was
significantly higher in patients with add-on lipid modulators,
pitavastatin and EPA (Figure 1B, C).
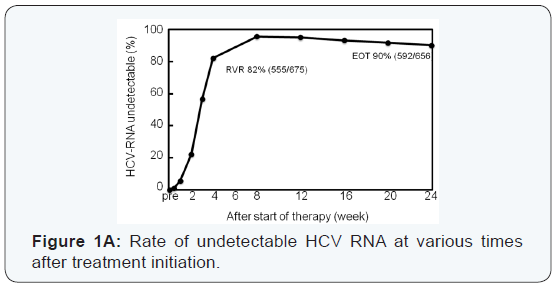
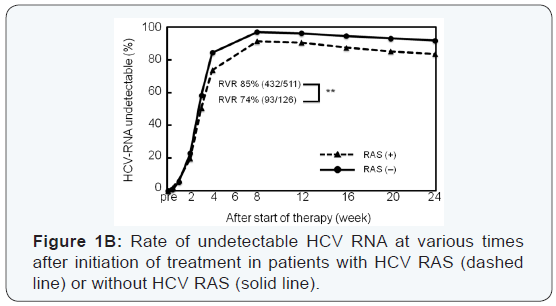
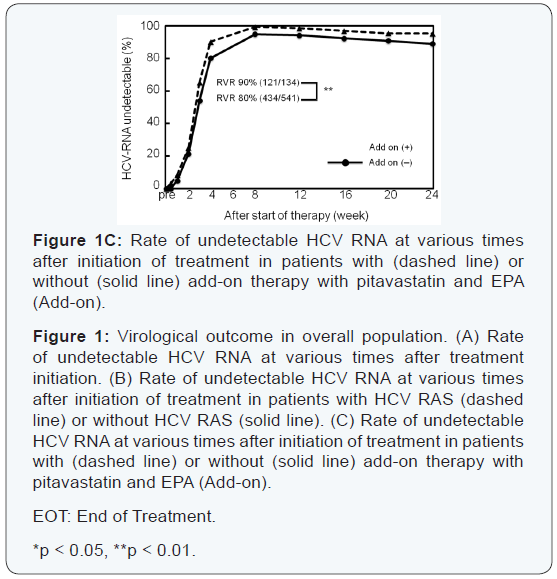
Thirty-eight patients experienced viral breakthrough. The
timing of viral breakthrough was spread over each treatment
period, and no specific trend was found. Eighteen patients with
viral breakthrough had HCV with RAS in NS3 D168, NS5A L31,
or NS5A Y93 at baseline, and six patients had prior exposure to
the protease inhibitor SMV. Twenty-one patients relapsed: 11 at
post-treatment week 4, eight at post-treatment week 12, and two
at post-treatment week 24. Ten patients who relapsed had HCV
with RASs, but only one patient with a history of SMV treatment
had relapse (data not shown).
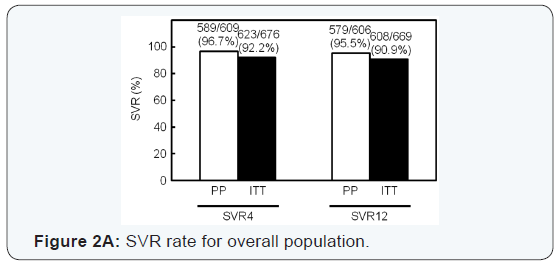
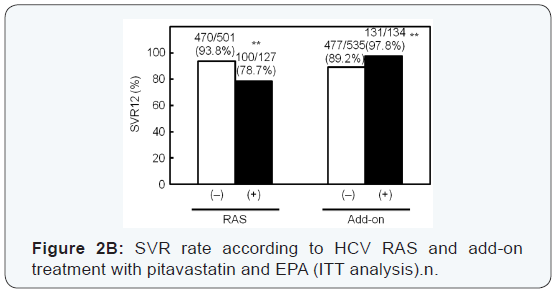
A total of 608 of 669 (91%) enrolled patients achieved SVR12
(Figure 2A). HCV RASs had the largest impact on SVR; 94% of
patients with HCV without RASs achieved SVR12, while SVR12
rate was only 79% for patients with HCV with RASs (Figure 2B).
Only five of 12 patients (42%) who had prior exposure to SMV
achieved SVR12; by contrast, SVR12 rate was high (97%) for
patients with a history of TVR treatment. Among patients who
had prior exposure to SMV, all five who had HCV with RASs at
baseline and two with HCV without RASs failed the treatment.
SVR12 rate for patients with pitavastatin and EPA was higher
(131/134, 98%) than that for patients with standard treatment
(477/535, 89%) (Figure 2B). Add-on treatment with pitavastatin
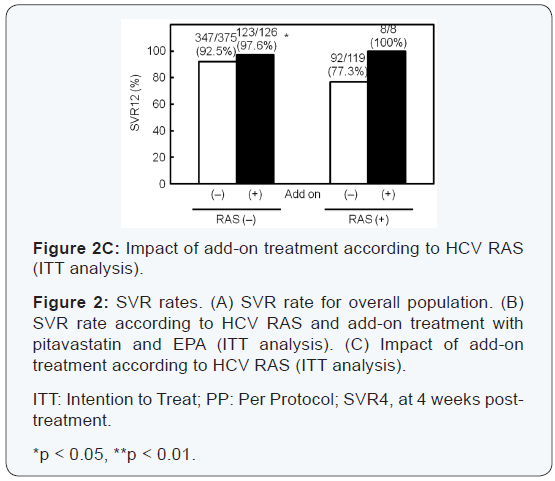
and EPA improved SVR12 rate for patients with HCV with RASs,
and all the patients achieved SVR12. SVR rate for the addon
group was also significantly higher than in patients in the
standard treatment group with HCV without RASs (Figure 2C).
SVR12 rate for patients with liver cirrhosis was significantly
lower than that of patients with chronic hepatitis; 228/261
(87%) versus 380/408 (93%). Age, sex, or previous treatment
response did not affect SVR12 rate. Patients with intractable
IL28B TG/GG genotype (rs8099917) showed a poor response at
weeks 3 and 4 of treatment; however, SVR12 rate was similar
between patients with IL28B TT and TG/GG (data not shown).
Predictive factors associated with SVR
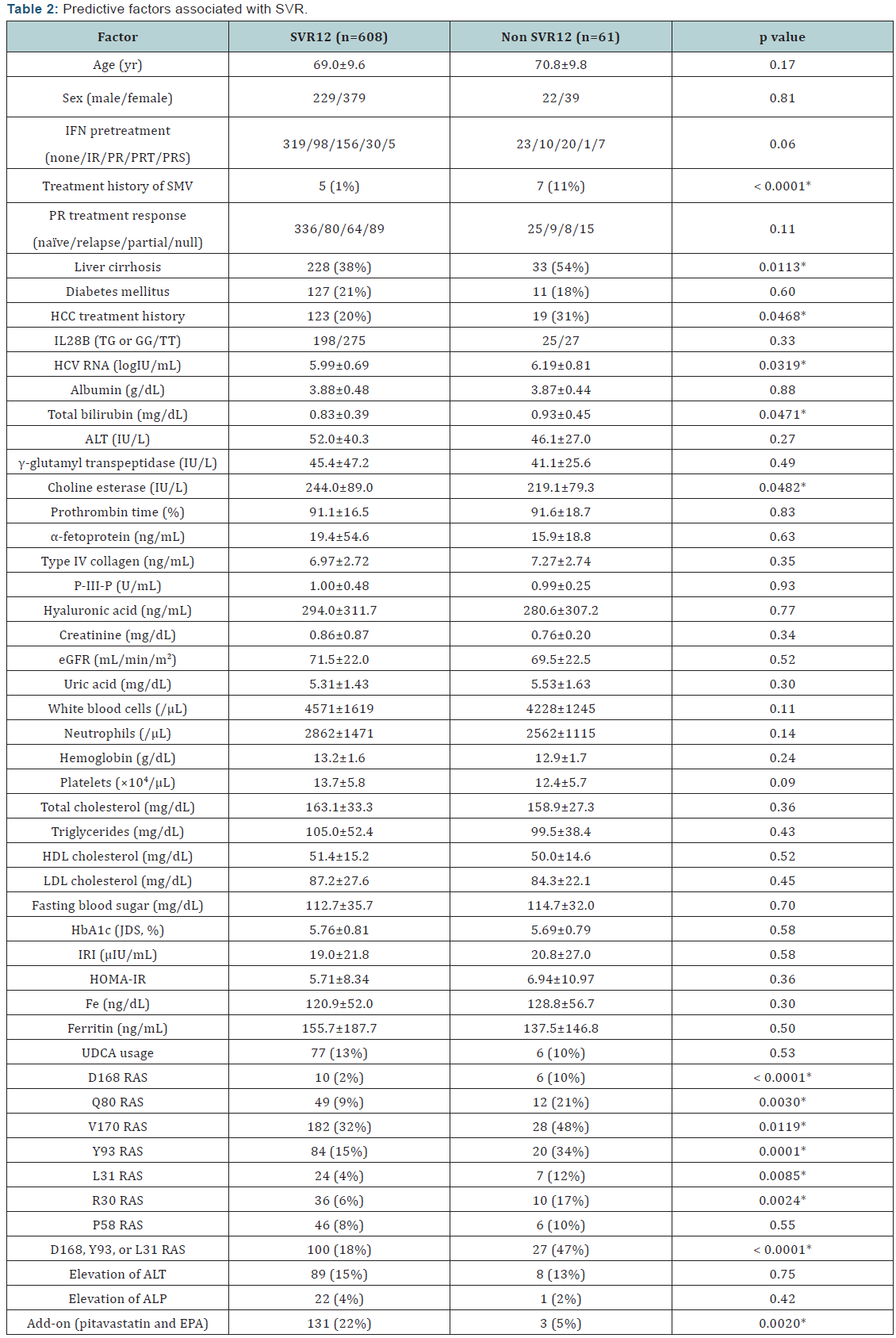
Predictive factors associated with SVR were examined
in all patients (Table 2). Univariate analysis identified some
parameters that correlated significantly with SVR12: prior
exposure to SMV (p<0.0001); liver cirrhosis (p=0.0113); HCV
RNA at baseline (p=0.0319); serum total bilirubin (p=0.0471);
serum choline esterase (p=0.0482); RAS at NS3 D168 (p<0.0001);
RAS at NS3 Q80 (p=0.003); RAS at NS3 V170 (p=0.0119); RAS
at NS5A Y93 (p=0.0001); RAS at NS5A L31 (p=0.0085); RAS at
NS5A R30 (p=0.0024); RAS at NS3 D168, NS5A L31, or NS5A
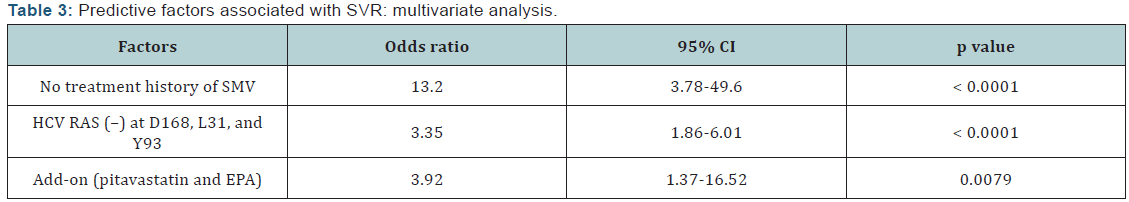
Y93 (p<0.0001); and add-on therapy (p=0.002). In multivariate
logistic regression analysis, significant contributory factors for
SVR12 were no prior exposure to SMV (OR 13.2, p<0.0001), no
RAS at NS3 D168, NS5A L31, or NS5A Y93 (OR 3.35, p<0.0001),
and add-on treatment with pitavastatin and EPA (OR 3.92,
p=0.0079) (Table 3).
Adverse events
Adverse events were evaluated according to the Common
Terminology Criteria for Adverse Event Version 4.0. The
frequently reported adverse events were ALT elevation, alkaline
phosphatase (ALP) elevation, pyrexia, headache, malaise, and
skin eruption. One hundred patients developed ALT elevation
(≥Grade 2), and 24 ALP elevation (≥Grade 2). Pyrexia and
headache were Grade 1. The frequencies of adverse events were
similar between patients with liver cirrhosis and those with
chronic hepatitis, and unaffected by age.
Discussion
In this study, HCV RNA rapidly became undetectable after
initiation of all-oral treatment with DCV and ASV, and 82% of
patients achieved RVR. A high SVR rate of 90.9% was achieved
after 24 weeks all-oral DAA treatment, which is clearly higher
than previous IFN-based antiviral treatments [4,5,17-20]. Viral
clearance was not affected by age, sex, previous treatment
response, or IL28B single nucleotide polymorphisms, which
were known to influence the outcome of IFN-based antiviral
treatment.
The rate for cessation of DCV and ASV treatment was low
(40 cases, 6%) and the severity of adverse events was mild.
Frequently reported adverse events were ALT elevation, ALP
elevation, pyrexia, headache, malaise, and skin eruption, as
published previously [24-29], and the frequency of adverse
events was not affected by age or liver fibrosis. Hence, this alloral
DAA treatment could be an adequate option for older or
cirrhotic patients. The highest complication during DCV and
ASV treatment was ALT elevation (100 cases, 14%), and 11 had
terminated therapy. Body weight was positively associated with
ALT elevation during DCV and ASV combination therapy (data
not shown), suggesting that serum and hepatic concentrations of ASV are involved in liver damage during therapy. Likewise,
patients with liver cirrhosis had a significantly higher risk of ALP
elevation (data not shown). DCV and ASV are mainly metabolized
in the liver [30], and it is possible that metabolism of DCV and/
or ASV in hepatocytes is involved in ALP elevation during DAA
treatment.
HCV with RASs at NS3 D168, NS5A L31, or NS5A Y93 were
detected in 131 patients in our study, and SVR rate for patients
with HCV with RASs was significantly lower than in those with
wild-type virus (Figure 2). However, the SVR rate for patients
with HCV with RASs was clearly higher than in the previous study
[29]. This might be partly because the physician tried to avoid
discontinuation of the treatment when encountering adverse
events. By sequencing the HCV RASs in patients who failed the
treatment, multiple HCV RASs were detected in most patients
with virological failure (data not shown), as previously reported
[26,29]. Furthermore, no RAS at NS3 D168, NS5A L31, or NS5A
Y93 before treatment was selected as one of the independent
significant contributory factors for SVR in multivariate logistic
regression analysis. Recently, fixed-dose treatment with
ledipasvir and sofosbuvir in patients with HCV genotype 1b
achieved a higher SVR rate than DCV and ASV treatment, even for
patients with HCV with RASs [31]. Examination for pretreatment
RAS and selection of patients might be important for future
adoption of treatment with DCV and ASV.
Prior exposure to SMV was also involved in response to
DCV and ASV treatment. SMV and ASV are both NS3/4 protease
inhibitors of the second generation and have similar therapeutic
and resistance profiles [23]. Therefore, even patients with HCV
without RASs who had prior SMV treatment might be predisposed
to acquire HCV with RASs for ASV. Thus, we need to take history
of treatment with the same DAA or ones with identical drug
potency into consideration for future DAA treatment.
In the present study, patients with advanced fibrosis achieved
a significantly lower SVR rate compared with those with chronic
hepatitis; however, this association disappeared in multivariate
analysis (Tables 2 & 3). Thus, liver fibrosis could affect the
outcome of DCV and ASV treatment for the following reasons. (1)
Drug delivery: patients with liver cirrhosis had portosystemic or
intrahepatic shunt and drug delivery could be inhibited and/or
drug concentration reduced in some part of the liver. (2) Immune
response: immunological disorder was observed in patients with
advanced liver fibrosis. Some patients with advanced liver fibrosis
with esophageal varices did not achieve SVR, and insufficient
drug delivery into the cirrhotic liver could affect viral clearance.
Furthermore, patients with intractable IL28B genotype showed
a poor response to treatment with DCV and ASV at weeks 3 and
4, suggesting that immune response might have some impact on
viral clearance during DCV and ASV treatment.
Previous studies on hepatic lipid metabolism have shown
that, in the liver with HCV infection, synthesis of cholesterol
and fatty acids is still activated, regardless of over-accumulation
of lipids [32-35]. Suppressive effects against HCV replication
by statins and EPA, and their synergistic action with IFN have
already been demonstrated in some HCV replicon systems [36-
39,41-46]. PUFAs including EPA inhibit HCV replication, which
may be independent of the regulating lipogenesis [38,39]. In
this study, patients with add-on therapy of pitavastatin and EPA
showed a significantly higher biological response rate to DCV
and ASV compared with those without add-on treatment (Figure
2). Negative modulation of lipid synthesis by pitavastatin and
EPA, activation of the antiviral host response to HCV infection
by pitavastatin, repression of HCV entry/infection through
suppression of low-density lipoproteins receptor expression by
EPA is thought to be involved in viral clearance during peg-IFN/
RBV treatment [46]. The mechanism of action is independent
of the antiviral effect of DAAs [30]. Therefore, addition of
pitavastatin and EPA could be useful to enhance the antiviral
effect on HCV infection via different mechanisms during future
DAA treatment. For reliable assessment, further accumulation of
clinical data is necessary for patients treated with this add-on
therapy.
Response of HCV RNA to treatment with DCV and ASV
was sufficiently favorable, even for women, older patients,
patients with liver cirrhosis or patients with intractable IL-28B
polymorphisms. HCV RASs and history of treatment with SMV
could be involved in antiviral host response and viral clearance.
Moreover, the lipid modulators, pitavastatin and EPA, enhanced
the efficacy of all-oral DCV and ASV combination therapy
through synergistic antiviral effect. It is possible that addition of
pitavastatin and EPA is effective for patients with treatmentresistant
HCV, which may increase SVR rates in patients treated
with DAAs in the future.




Comments
Post a Comment